Analysis on the Clinical Research Progress of RAS antagonists
The Renin-Angiotensin System (RAS) is a crucial fluid-regulating system composed of a series of peptide hormones and corresponding enzymes. It is involved in regulating arterial blood pressure and plasma sodium concentration, maintaining the balance of blood pressure, water, and electrolytes in the human body, and maintaining the relative stability of the body's internal environment. The Renin-Angiotensin System has various dual regulatory effects and homeostatic regulation functions within and outside the system, promoting tissue reconstruction and resisting pathologic reconstruction. It plays important roles not only in hypertension, kidney diseases, and myocardial hypertrophy but also in almost all cardiovascular and cerebrovascular diseases. Over activation of the Renin-Angiotensin-Aldosterone System is one of the reasons for the development of hypertension.
RAS antagonists include Angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs, sometimes known as sartans) and direct renin inhibitors (eg, aliskiren).
ACE inhibitors: Benazepril Hydrochloride
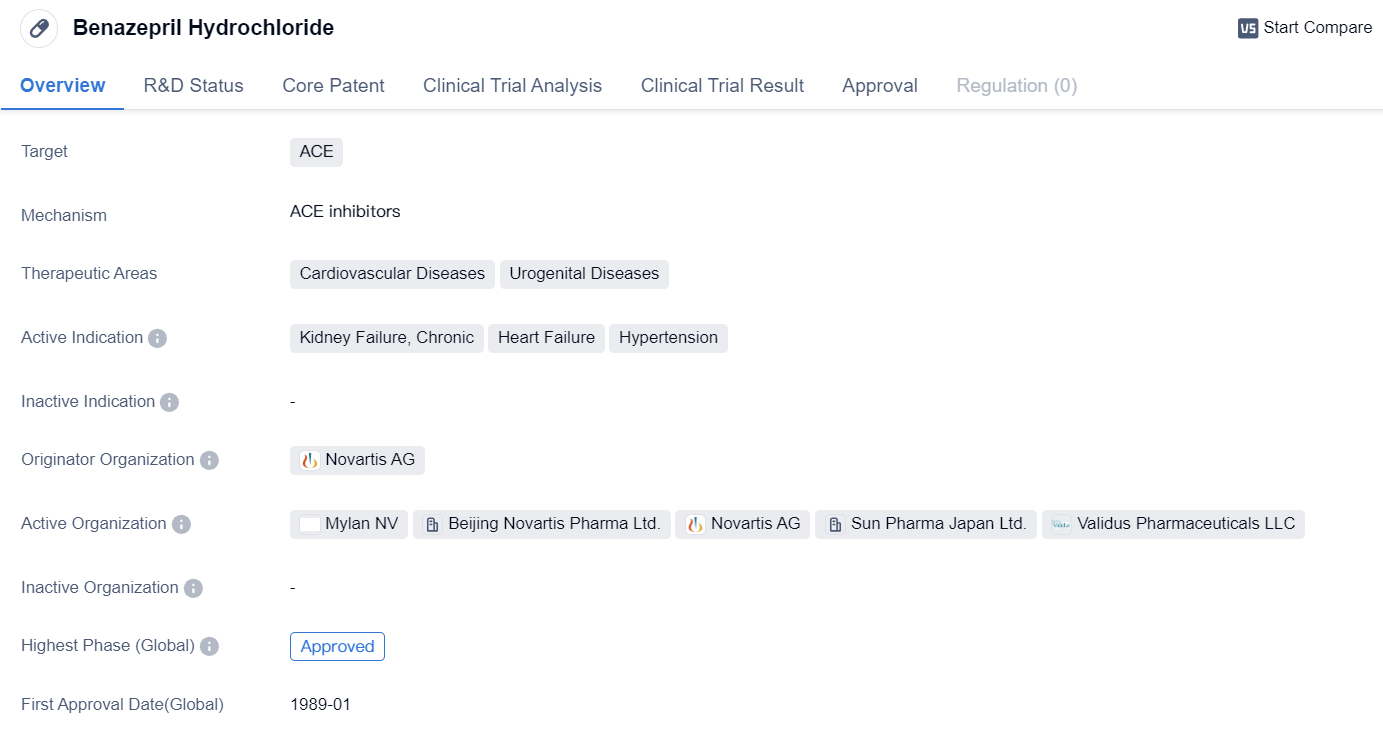
Benazepril Hydrochloride is a small molecule drug that belongs to the class of ACE inhibitors. It is primarily used in the treatment of cardiovascular diseases and urogenital diseases. The drug targets ACE, an enzyme involved in the regulation of blood pressure and fluid balance.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The therapeutic areas in which Benazepril Hydrochloride is indicated include kidney failure, chronic heart failure, and hypertension. These conditions are prevalent worldwide and pose significant health risks to patients. By inhibiting ACE, Benazepril Hydrochloride helps to relax blood vessels, reduce fluid retention, and lower blood pressure, thereby improving the symptoms associated with these diseases.
The drug was first approved globally in January 1989, indicating its long-standing presence in the pharmaceutical market. Its approval in China also signifies its availability and acceptance in one of the largest pharmaceutical markets in the world.
Benazepril Hydrochloride was developed by Novartis AG, a renowned pharmaceutical company known for its contributions to the healthcare industry. Novartis AG is a global leader in the development and commercialization of innovative medicines, with a strong focus on cardiovascular and renal diseases.
The fact that Benazepril Hydrochloride has reached the highest phase of approval both globally and in China highlights its efficacy and safety profile. This indicates that extensive clinical trials and regulatory assessments have been conducted to ensure its effectiveness and minimal adverse effects.
In summary, Benazepril Hydrochloride is a small molecule drug developed by Novartis AG. It targets ACE and is primarily used in the treatment of cardiovascular diseases and urogenital diseases. Its active indications include kidney failure, chronic heart failure, and hypertension. With its global and China approvals dating back to 1989, Benazepril Hydrochloride has established itself as a trusted and widely available medication for patients suffering from these conditions.
ARBs: Azilsartan
Azilsartan is a small molecule drug that targets the angiotensin II type 1 receptor (AT1R) and is primarily used in the treatment of cardiovascular diseases, specifically hypertension. It was developed by Takeda Pharmaceutical Co., Ltd., a renowned pharmaceutical organization. The drug has successfully completed the highest phase of clinical trials and has received approval both globally and in China.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
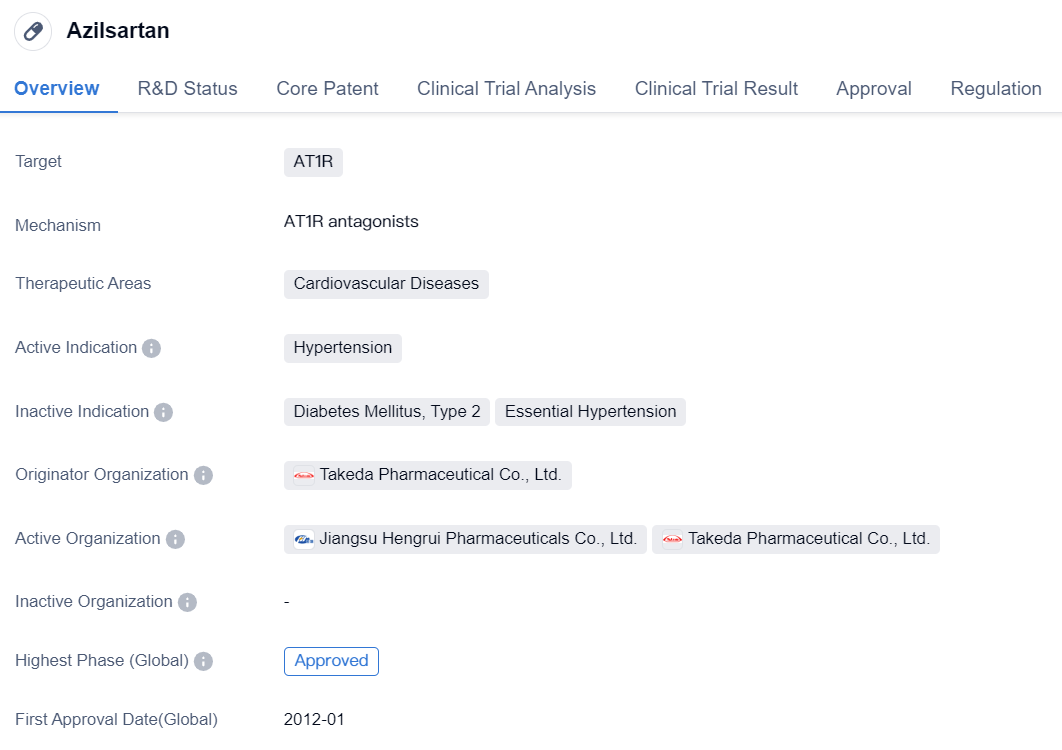 Azilsartan was first approved in Japan in January 2012, making it the country of origin for this drug. The approval process for Azilsartan was expedited through priority review, indicating its potential significance in addressing the medical needs of patients suffering from hypertension.
Azilsartan was first approved in Japan in January 2012, making it the country of origin for this drug. The approval process for Azilsartan was expedited through priority review, indicating its potential significance in addressing the medical needs of patients suffering from hypertension.
As a small molecule drug, Azilsartan is designed to interact with the AT1R receptor, which plays a crucial role in regulating blood pressure. By targeting this receptor, Azilsartan helps to block the binding of angiotensin II, a hormone that causes blood vessels to constrict, leading to increased blood pressure. By inhibiting this process, Azilsartan effectively lowers blood pressure and reduces the risk of cardiovascular complications associated with hypertension.
The approval of Azilsartan in both global and Chinese markets highlights its efficacy and safety profile. This drug has undergone rigorous clinical trials to demonstrate its therapeutic benefits and has met the necessary regulatory requirements for approval. The fact that Azilsartan has reached the highest phase of clinical development indicates that it has undergone extensive testing and evaluation, providing confidence in its effectiveness and safety.
In summary, Azilsartan is a small molecule drug developed by Takeda Pharmaceutical Co., Ltd. It targets the AT1R receptor and is primarily used for the treatment of hypertension, a common cardiovascular disease. With its approval in Japan and China, Azilsartan has demonstrated its efficacy and safety in clinical trials, making it a valuable option for patients suffering from hypertension. The priority review designation further emphasizes the importance of this drug in addressing the medical needs of patients and highlights its potential impact in the field of biomedicine.
Direct renin inhibitors: Aliskiren Fumarate
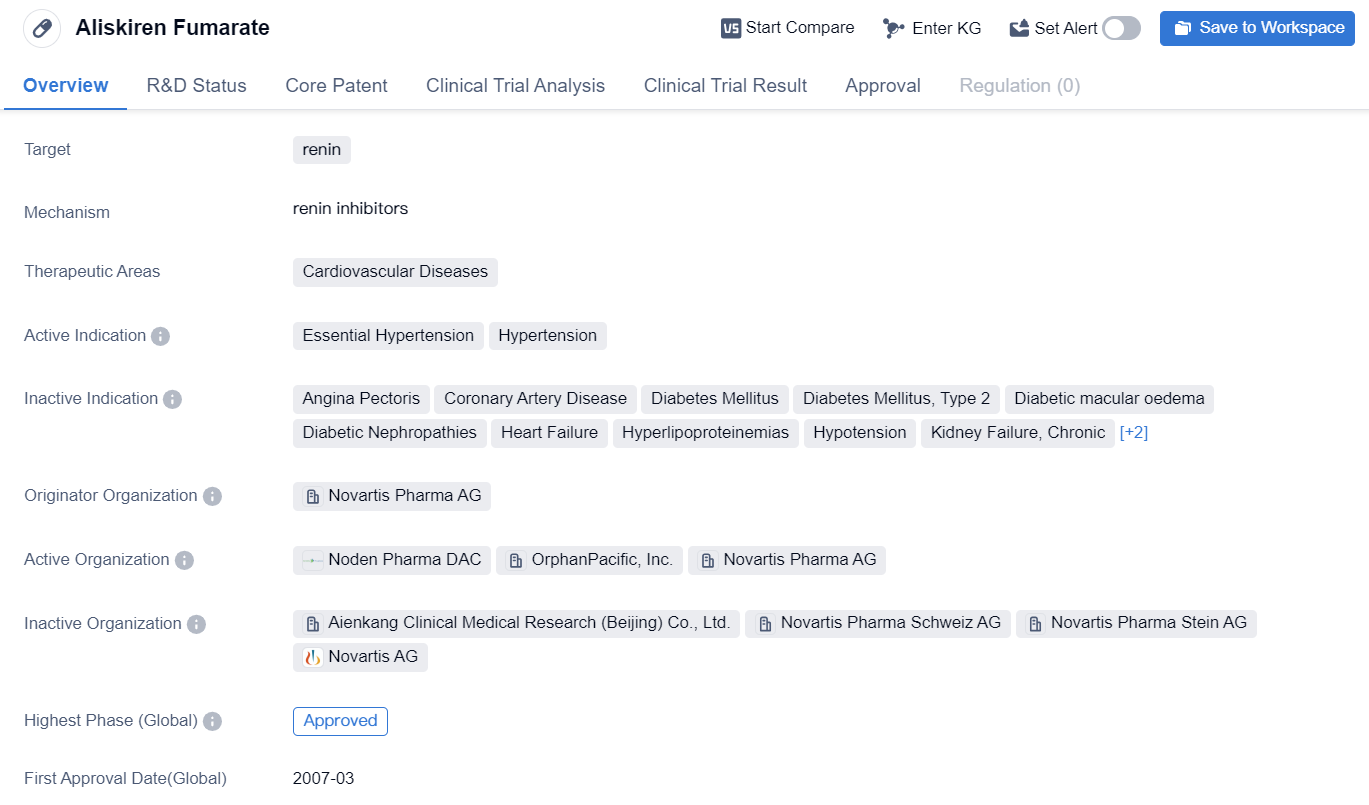
Aliskiren Fumarate is a small molecule drug that targets renin, an enzyme involved in the regulation of blood pressure. It is primarily used in the treatment of cardiovascular diseases, specifically essential hypertension and hypertension. The drug was developed by Novartis Pharma AG, a leading pharmaceutical company.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Aliskiren Fumarate has received approval for use in multiple countries, including the United States. Its first approval date in the global market was in March 2007. The drug has been widely accepted and is currently in the highest phase of development, which is the approved phase, both globally and in China.
Essential hypertension, also known as primary hypertension, is a chronic medical condition characterized by high blood pressure with no identifiable cause. It is a common condition affecting a significant portion of the population. Hypertension, on the other hand, refers to high blood pressure resulting from various underlying causes.
Aliskiren Fumarate works by inhibiting renin, an enzyme involved in the production of angiotensin II, a hormone that constricts blood vessels and increases blood pressure. By blocking the action of renin, the drug helps to relax blood vessels, reduce blood pressure, and improve cardiovascular health.
The approval of Aliskiren Fumarate in multiple countries, including the United States, highlights its efficacy and safety profile. It has undergone rigorous clinical trials to demonstrate its effectiveness in treating essential hypertension and hypertension. The drug's approval in China further expands its market reach and potential patient population.
As a small molecule drug, Aliskiren Fumarate offers advantages such as ease of formulation, administration, and manufacturing. These factors contribute to its widespread use and availability in the market.
In summary, Aliskiren Fumarate is a small molecule drug developed by Novartis Pharma AG that targets renin to treat cardiovascular diseases, specifically essential hypertension and hypertension. Its approval in multiple countries, including the United States and China, signifies its efficacy and safety. The drug's mechanism of action involves inhibiting renin to reduce blood pressure and improve cardiovascular health.