Sandoz's Tyruko® (natalizumab), a unique biosimilar for multiple sclerosis, has been approved by the European Commission
Sandoz, an international pioneer in the field of generic and biosimilar drugs, shared news that the EC green-lighted marketing approval for the unique biosimilar Tyruko® (natalizumab), created by Polpharma Biologics, making it the first of its kind to obtain such authorization.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
This approval incorporates the therapy as a standalone disease-altering treatment for adults suffering from highly active RRMS, mirroring the authorization granted by the EC for the Tysabri® (natalizumab) benchmark drug.
According to Rebecca Guntern, Sandoz’s President in Europe, the absence of a cure for the chronic disease that is multiple sclerosis necessitates immediate access to affordable, high-grade healthcare. She opined that this recent authorization brings us incrementally closer to alleviating the hardships of MS for patients across Europe by increasing availability of much-needed, life-improving treatments.
Multiple sclerosis (MS) is classified as a persistent inflammatory and neurodegenerative disorder of the central nervous system that can massively impair one's routine activities. Majority of those affected by MS experience episodes of fresh or intensifying symptoms, referred to as relapses, which are subsequently followed by periods of disease reprieve, wherein symptoms can either partially or fully improve. Initiating treatment with DMTs early on can alter the trajectory of a patient's MS, making future disability less likely.
In 2019, Sandoz and Polpharma Biologics entered into a global pact for the commercial release of biosimilar natalizumab. Under the terms of this agreement, Polpharma Biologics retains responsibilities for drug development, manufacturing, and supply. Having gained an exclusive global license, Sandoz now has the authority to commercialize the drug and oversee its distribution across all markets.
Driven by their mission to provide broad access to critical and potentially life-altering biologic drugs in a sustainable and cost-effective manner, Sandoz targets multiple areas including immunology, oncology, supportive care, and endocrinology. Their global portfolio is quite impressive, boasting eight marketed biosimilars. Having introduced the first biosimilar in Europe back in 2006, Sandoz has since then championed early and broadened patient access to transformative drugs, thereby enhancing healthcare savings and promoting competition that stimulates further innovation.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
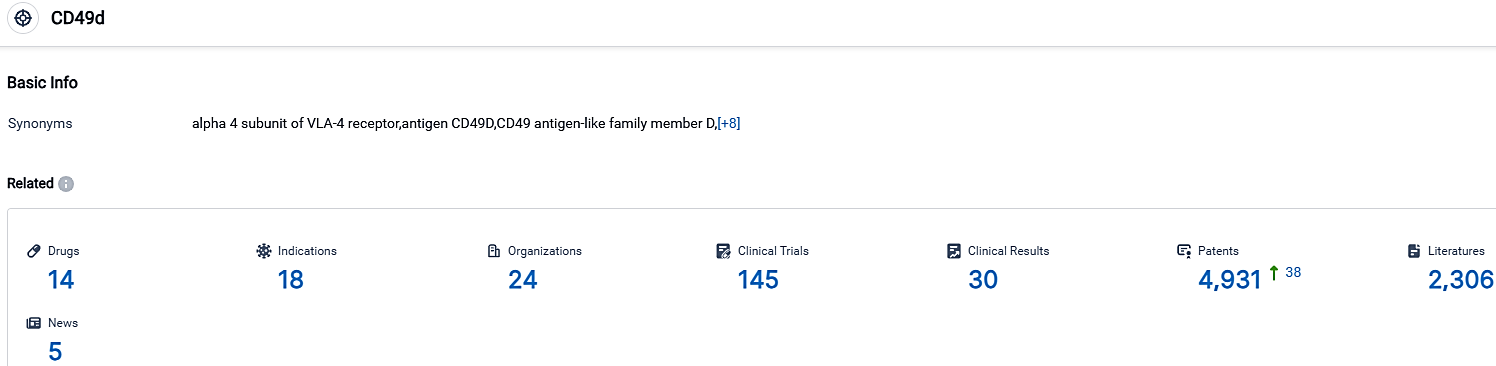
According to the data provided by the Synapse Database, As of September 30, 2023, there are 14 investigational drugs for the CD49d target, including 18 indications,24 R&D institutions involved, with related clinical trials reaching 145,and as many as 4931 patents.
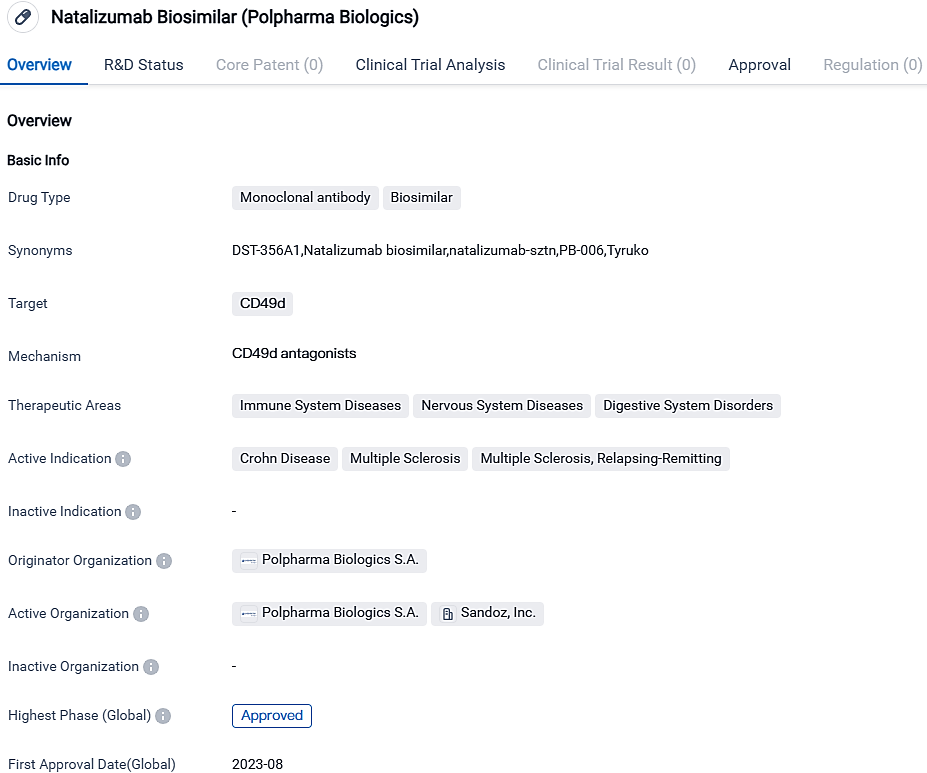
Natalizumab Biosimilar, a cloned antibody biosimilar medicine, zeroes in on CD49d. The medication is prescribed for Crohn Disease, Multiple Sclerosis, and Relapsing-Remitting Multiple Sclerosis therapy. The United States granted its initial approval for Natalizumab Biosimilar in August 2023. In the EU, Tyruko is approved as a solitary disease-altering treatment for adults battling highly active relapsing-remitting multiple sclerosis.