Exploring Palbociclib: A Versatile Therapeutic in Cancer Treatment and Beyond
Palbociclib was first approved in the United States in February 2015 and has since been approved in other countries around the world. It has received various regulatory designations, including priority review, accelerated approval, breakthrough therapy, and special review project.
Palbociclib is a small molecule drug that targets CDK4 and CDK6 and is used for the treatment of various therapeutic areas, including neoplasms, skin and musculoskeletal diseases, immune system diseases, nervous system diseases, cardiovascular diseases, congenital disorders, digestive system disorders, endocrinology and metabolic disease, hemic and lymphatic diseases, respiratory diseases, urogenital diseases, and other diseases.
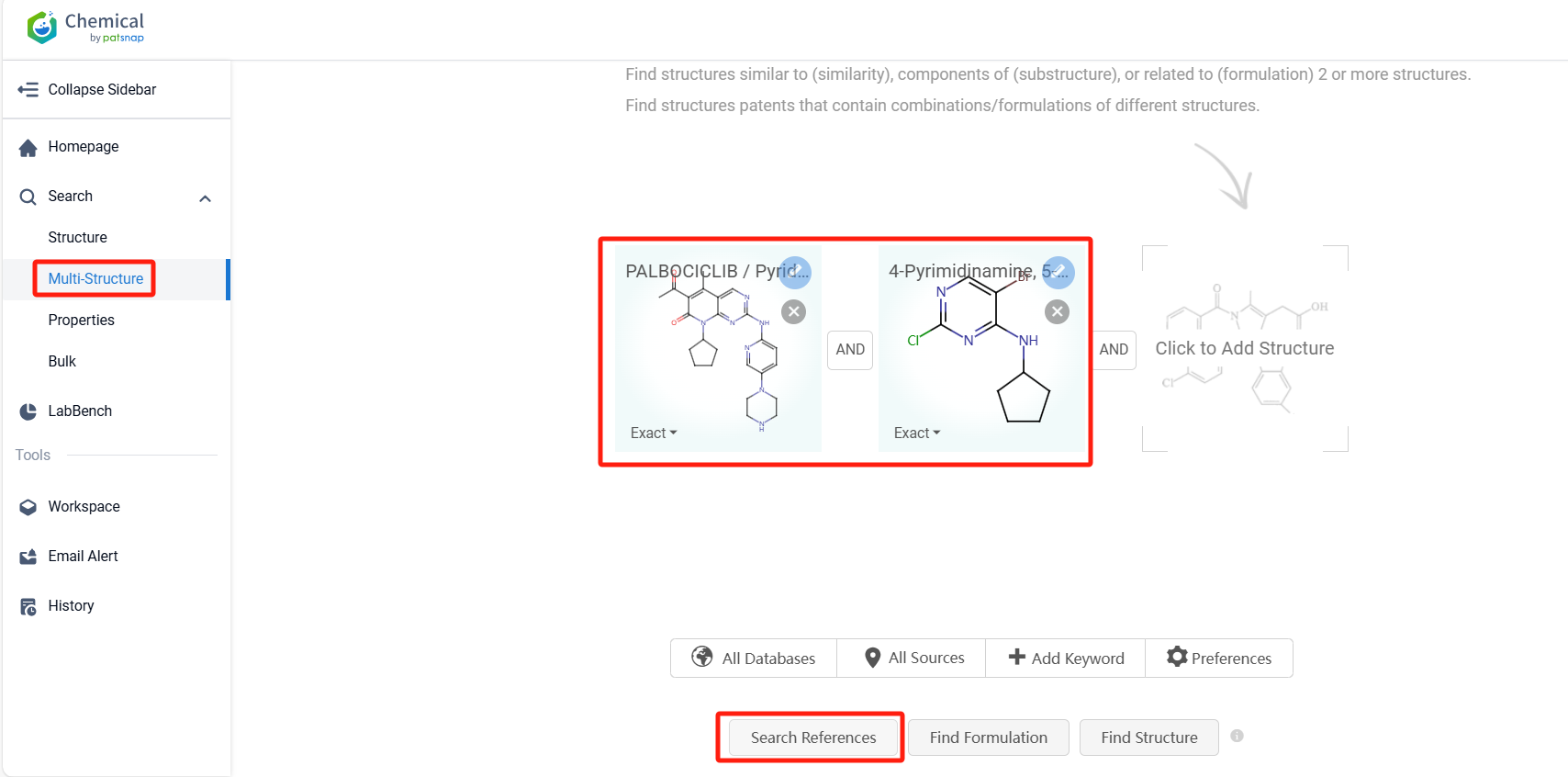
Log in to the Patsnap Chemical. Select the Multi-Structure search, and search for the reactants and products in the specific steps of the synthesis route of Palbociclib. click on search references, and you can query the relevant literature.
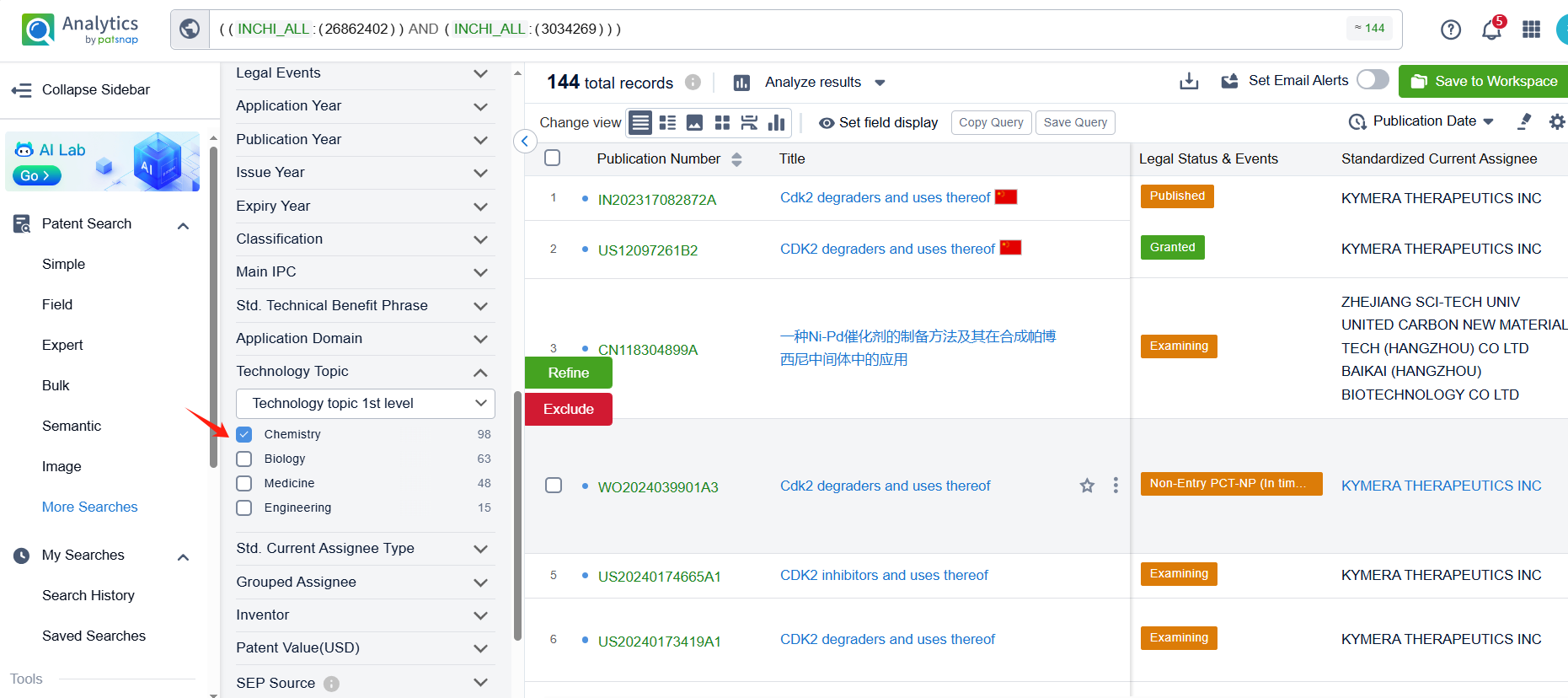
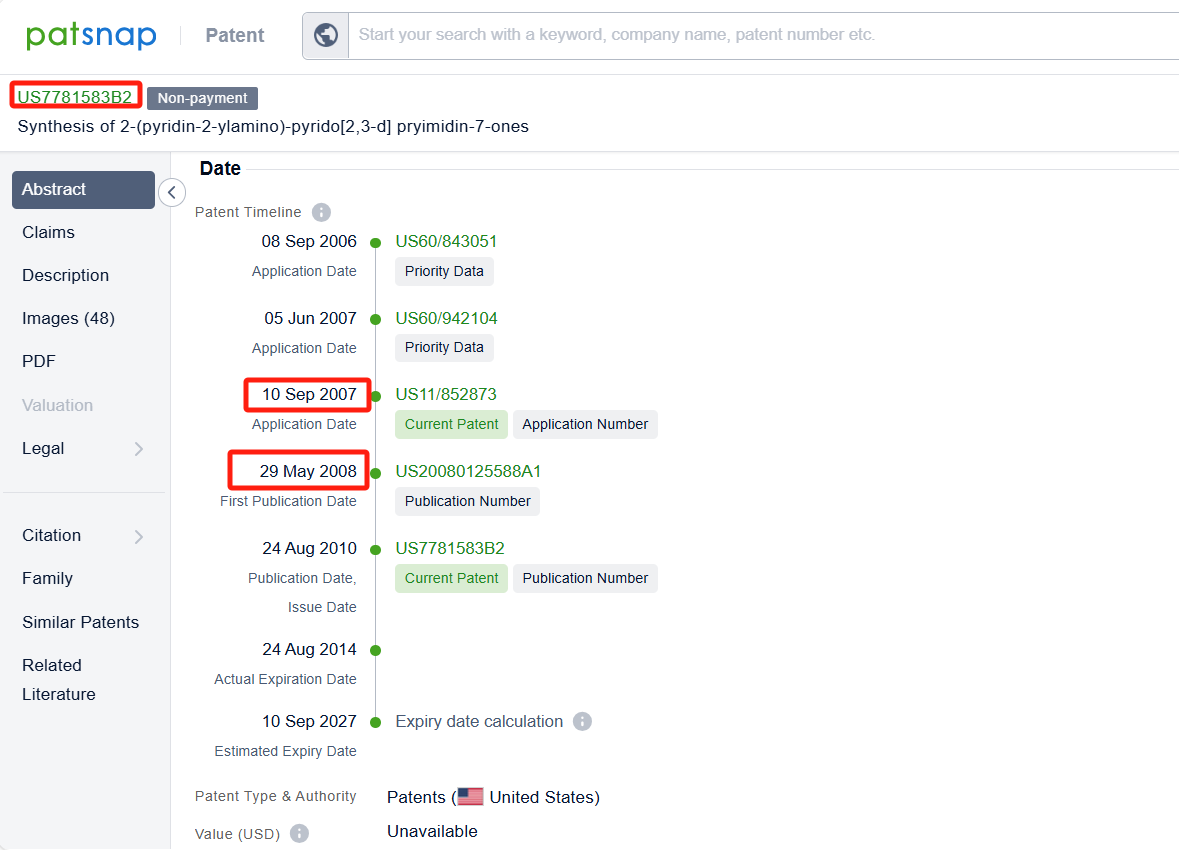
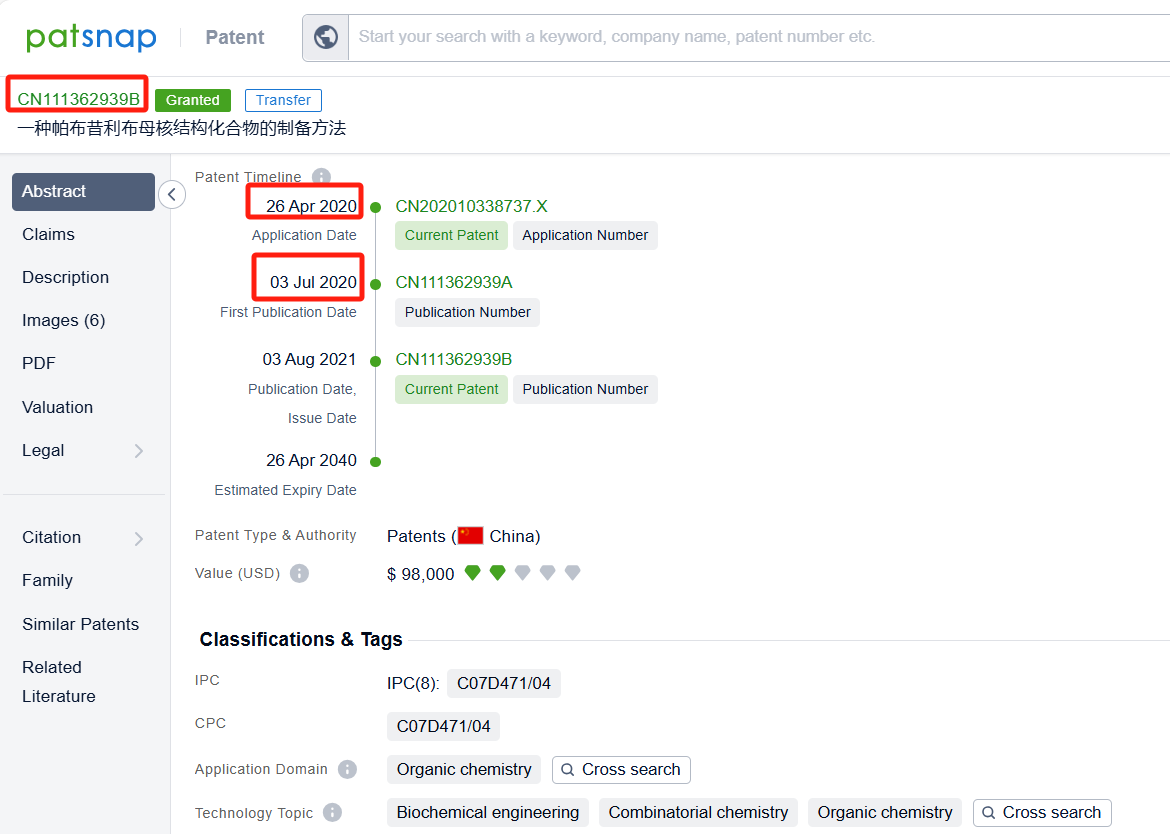
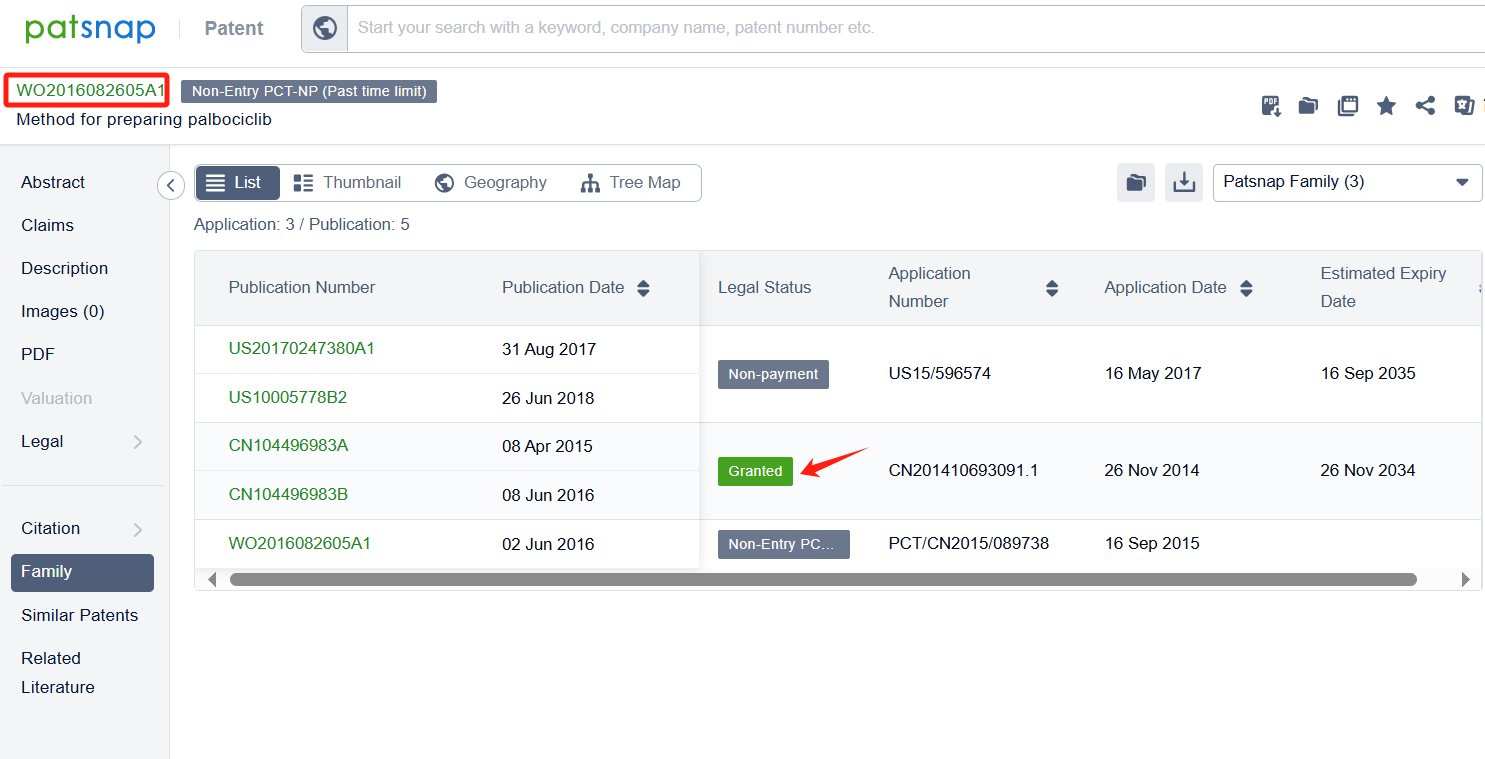
Linked to the Patsnap patent, check the 'Chemistry' category under the technical subject classification and click on the filter, which will allow you to accurately retrieve the design and improvement of the Palbociclib process route by Pfizer Inc. and other companies,Such as Pfizer Inc.'s patent US7781583B2 (application date 20070910, publication date 20080529) describes a new method for making 2-(pyridin-2-ylamino)-pyrido[2,3-d] pryimidin-7-ones, The method involves reacting certain compounds with each other in the presence of a transition metal catalyst, a base, and optionally a phosphine agent. Shandong Zhongke Taidou Chemical Co., Ltd's patent CN111362939B (application date 20200426, publication date 20200703) describes a method for preparing palbociclib parent core structural compound using cytosine as a starting material, with a total process yield of more than 65% and a purity of more than 99.3%. The patent was granted on August 3, 2021, with an expiration date of April 26, 2040. Additionally, Suzhou Miracpharma Technology Co., Ltd.'s patent WO2016082605A1(application date 20150916, publication date 20160602) provide a simple and economical method for preparing Palbociclib. Its corresponding patents in China and the United States have all been granted.
The drug was developed by Pfizer Inc. and has reached the highest phase of approval both globally and in China. Palbociclib is indicated for use in a wide range of cancer types and has shown promise in treating various types of advanced and recurrent cancers. Its approval in multiple countries and its regulatory designations suggest that it may offer significant benefits to patients with these types of cancers.
Overall, the diverse therapeutic areas and indications for Palbociclib, as well as its regulatory status, indicate its potential as an important treatment option for patients with various types of cancers and tumors. This drug is a key player in the pharmaceutical industry, particularly in the treatment of advanced and recurrent cancer.
AI built to maximize IP and R&D efficiency
Redefine chemical FTO with a range of structure retrieval options at your fingertips, from exact matches to similarity searches, all powered by deep data processing techniques and proprietary AI algorithms to eliminate the risk of omitting key results.