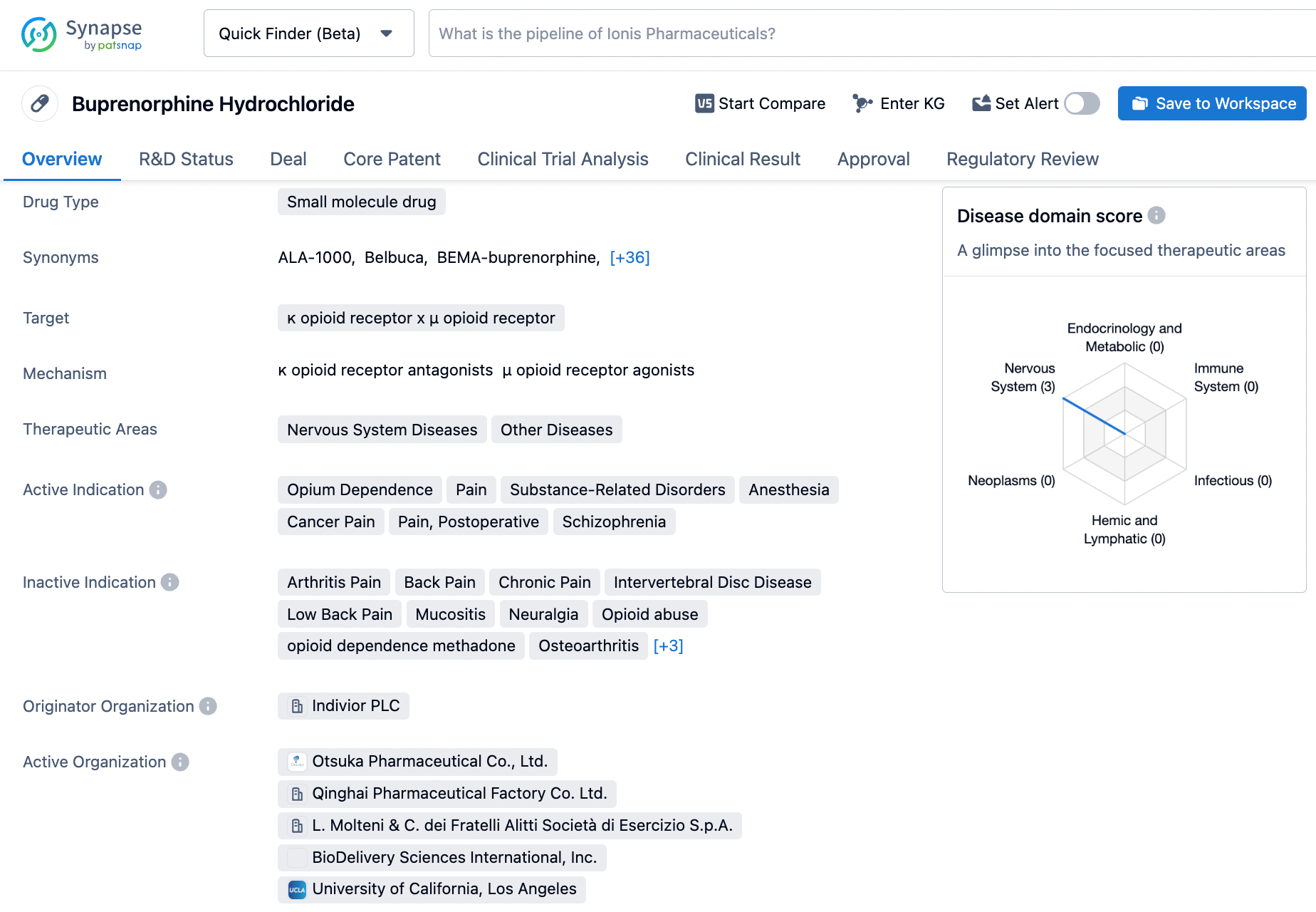
Optimize Your Synapse Experience: A Step-by-Step Guide to Searching Buprenorphine
Buprenorphine Hydrochloride, a drug approved in US on December 29, 1981, is manufactured by Indivior and is primarily used to manage pain that requires opioid analgesics and for which alternative treatments are insufficient. Buprenorphine works as a partial agonist at the mu-opioid receptor and as an antagonist at the kappa-opioid receptor. Its slow rate of dissociation from its receptor, which has been observed in vitro studies, accounts for its longer duration of action compared to morphine, its unpredictability of reversal by opioid antagonists, and its low level of manifest physical dependence. Buprenex is an important drug for pain management and has been widely used in the medical field. Click on the image below to begin the exploration journey of Buprenorphine through the Synapse database!
You can search for the latest pharmaceutical information such as drugs, targets, patents, transactions, clinical results, etc. through the Synapse database. Come and experience it!