Overview of Janssen’s Pipelines|R&D Progress
Janssen is a pharmaceutical company owned by Johnson & Johnson, a multinational company in the global medical device, pharmaceutical and consumer goods sectors. Based in New Jersey , The company has a diverse portfolio of drugs targeting various therapeutic areas. Janssen focuses on developing, manufacturing and marketing innovative medicines to meet the needs of patients around the world. The company is dedicated to the research and development of drugs to treat a variety of disease areas, including cardiovascular disease, neuroscience, infectious diseases, immune diseases, and oncology. Janssen has several research and development facilities around the world, and its products are distributed in hundreds of countries and territories around the world. As part of Johnson & Johnson, Janssen has been working to integrate science and technology with global health needs to improve people's quality of life. Through continuous innovative drug research, Janssen strives to provide safer and more effective treatment options for patients around the world.
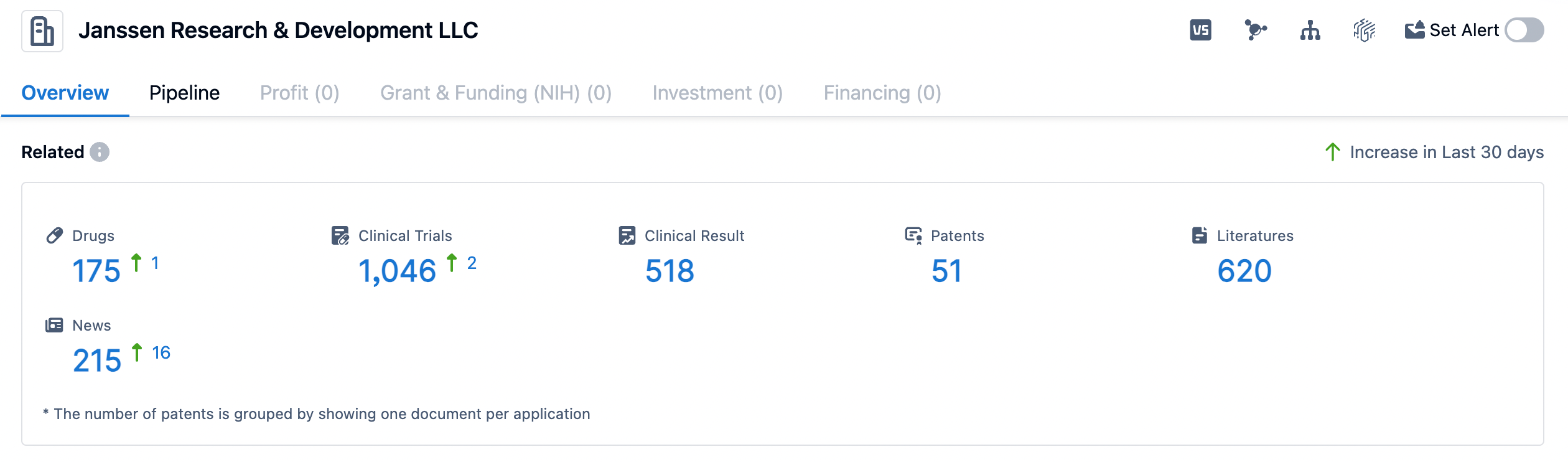
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of Janssen.
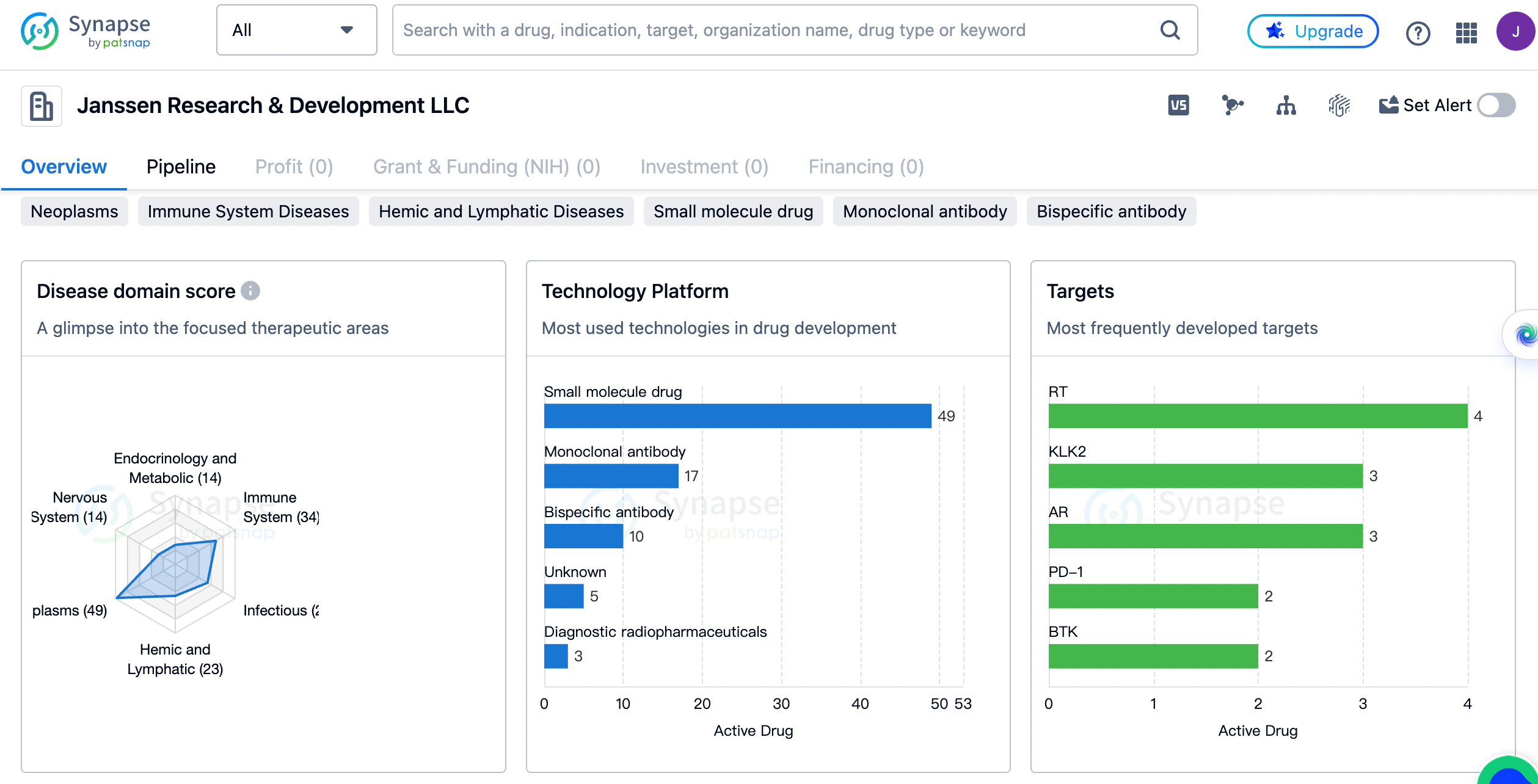
Janssen has developed drugs for a wide range of therapeutic areas. The highest number of drugs, 48 in total, are focused on the treatment of neoplasms, which are abnormal growths of tissue commonly known as tumors. This indicates that Janssen has a strong focus on oncology and is committed to developing innovative treatments for cancer patients.
The second highest number of drugs, 34 in total, are aimed at immune system diseases. This includes conditions such as autoimmune disorders, allergies, and immunodeficiency disorders. Janssen 's focus on immune system diseases highlights the importance of developing therapies that can modulate and regulate the immune system to treat various disorders.
Infectious diseases are also a significant area of focus for Janssen, with 26 drugs developed for this therapeutic area. This includes treatments for viral, bacterial, and fungal infections. The ongoing global pandemic caused by the COVID-19 virus has further emphasized the need for effective treatments and vaccines for infectious diseases.
Cardiovascular diseases (16 drugs), skin and musculoskeletal diseases (16 drugs), respiratory diseases (15 drugs), endocrinology and metabolic diseases (14 drugs), and nervous system diseases (13 drugs) are also areas of focus for Janssen. These therapeutic areas represent significant medical challenges and highlight the company's commitment to addressing unmet medical needs in these fields.
The most frequently developed targets by Janssen
The target with the highest number of drugs, 4 in total, is RT. RT, or reverse transcriptase, is an enzyme involved in the replication of retroviruses such as HIV. This indicates Janssen 's focus on developing antiretroviral therapies for the treatment of HIV/AIDS. Other frequently developed targets include KLK2 and AR, both with 3 drugs each. KLK2 is a biomarker associated with prostate cancer, while AR refers to androgen receptors, which play a crucial role in the development and progression of prostate cancer. The presence of multiple drugs targeting these specific biomarkers suggests Janssen 's dedication to advancing treatments for prostate cancer. PD-1, BTK, and HIV-1 RT are among the targets with 2 drugs each. PD-1 is a checkpoint protein involved in regulating the immune response, and drugs targeting PD-1 have revolutionized cancer treatment in recent years. BTK, or Bruton's tyrosine kinase, is a target for the treatment of B-cell malignancies such as chronic lymphocytic leukemia. HIV-1 RT, as mentioned earlier, is a target for antiretroviral therapy in HIV/AIDS.
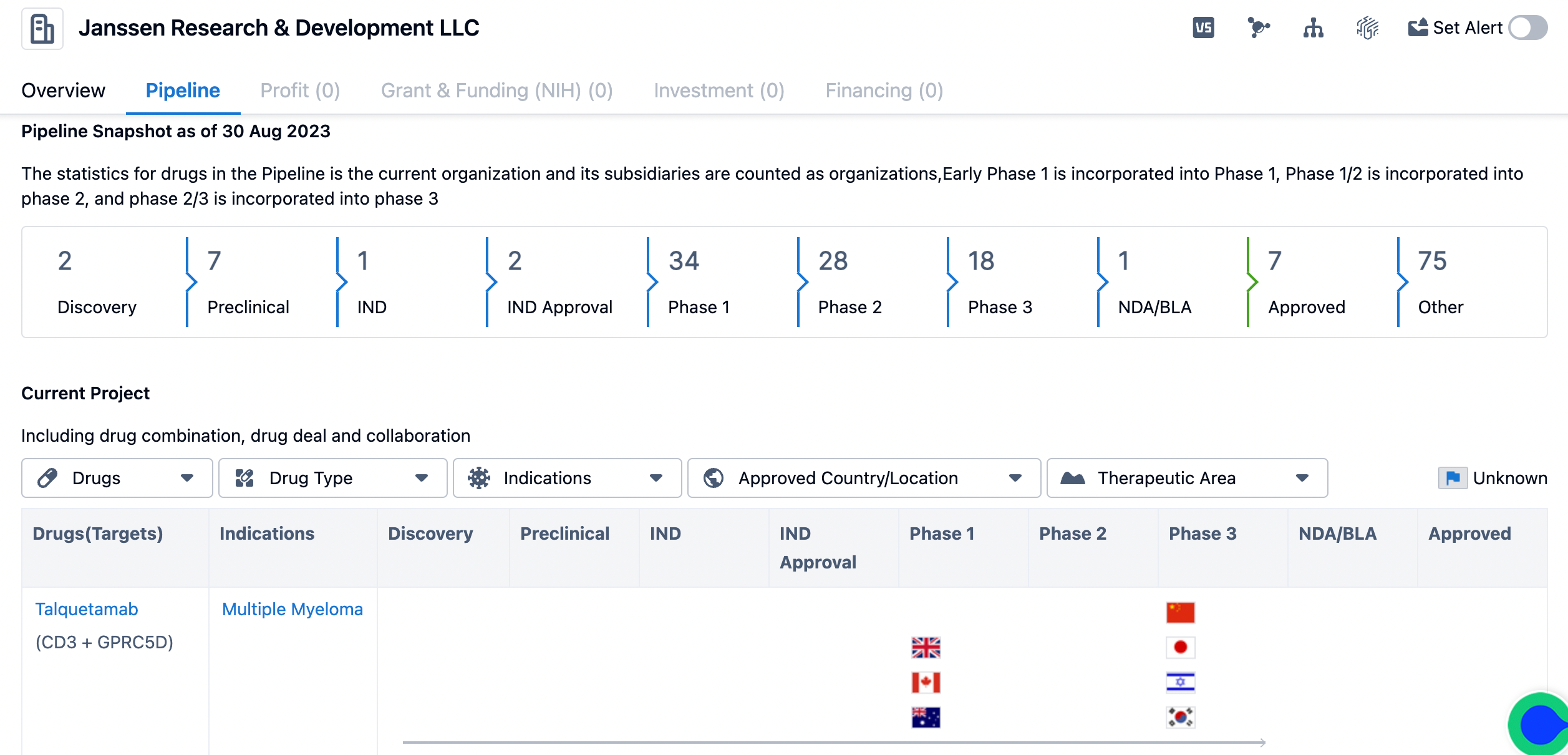
Pipeline of Janssen
The pipeline consists of drugs in various stages of development, starting from discovery to approved drugs. The majority of drugs are in the preclinical stage (7 drugs) and the phase 1 stage (34 drugs). This indicates that Janssen is actively exploring new drug candidates and conducting early-stage clinical trials.
The pipeline also includes drugs in the phase 2 stage (28 drugs) and phase 3 stage (18 drugs), which are more advanced stages of clinical development. These drugs have shown promising results in earlier stages and are being further evaluated for safety and efficacy in larger patient populations. The presence of drugs in these stages indicates that Janssen is progressing towards potential regulatory approval and commercialization. Additionally, the pipeline includes drugs in the IND (Investigational New Drug) stage (1 drug), IND Approval stage (2 drugs), NDA/BLA (New Drug Application/Biologics License Application) stage (1 drug), and approved stage (7 drugs). These stages represent important milestones in the drug development process, with IND approval allowing for clinical trials to proceed and NDA/BLA submission indicating readiness for regulatory review. It is worth noting that the pipeline also includes a significant number of drugs categorized as "Other" (75 drugs). This category may include drugs in early discovery stages, drugs targeting rare diseases, or drugs with alternative development pathways.
In summary, Janssen is a pharmaceutical organization with a strong focus on the development of drugs for various therapeutic areas. The company has a significant presence in oncology, immune system diseases, and infectious diseases. It is actively developing drugs targeting a wide range of biomarkers and has a diverse pipeline of drugs in different stages of development. Janssen's commitment to advancing healthcare is evident through its efforts to address unmet medical needs and improve patient outcomes.