European Commission approves Pfizer's LITFULO™ for Severe Alopecia Areata
Pfizer Inc. confirmed the received marketing approval by the EC for LITFULO™ (ritlecitinib) aimed for the treatment of severe alopecia areata in adults and adolescents aged 12 years and above. As a daily oral tablet, LITFULO symbolizes the first EC-sanctioned pharmaceutical directed towards the treatment of severe alopecia areata in individuals as young as 12 years old.
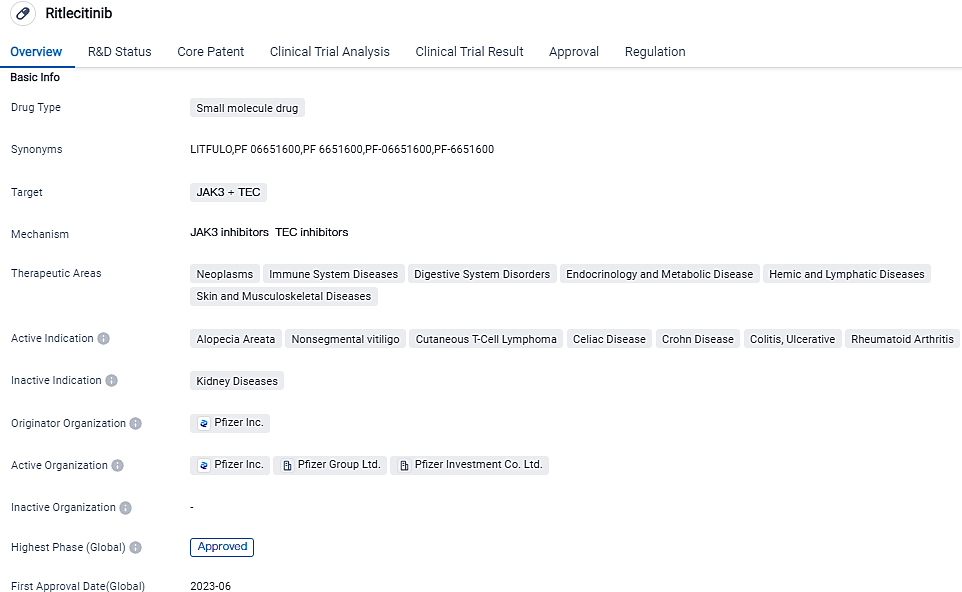
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
LITFULO stands out as the pioneering and sole therapy that specifically obstructs Janus kinase 3 (JAK3) and the tyrosine kinase identified in hepatocellular carcinoma (TEC) kinases family.
“Securing the European authorization for LITFULO is a significant advancement for patients as young as 12 suffering from substantial hair loss due to alopecia areata because it opens a gateway to potentially experience substantial hair regrowth," expressed Angela Hwang, Chief Commercial Officer, President, Global Biopharmaceuticals Business at Pfizer. “Previous to this, there were zero treatment options sanctioned by the EC for adolescents facing serious alopecia areata, and Pfizer is honored to introduce this innovative therapeutic solution for patients."
The market approval for LITFULO is recognized across the entire 27 EU member states, including Iceland, Liechtenstein, and Norway. This validation succeeds the recommendation for approval by the EMA Committee for Medicinal Products for Human Use in July 2023 and follows endorsements by the U.S. FDA and the Japanese Ministry of Health, Labour and Welfare in June 2023.
The approval developed from the ALLEGRO clinical trial program, which incorporated the ALLEGRO Phase 2b/3 study that evaluated LITFULO in patients 12 years and up suffering from alopecia areata. ALLEGRO-LT, a progressive Phase 3, open-label, long-term investigation, is collecting safety and efficacy data for adults. Long-term effectiveness and safety data gathered from this study supported the submission for the approval.
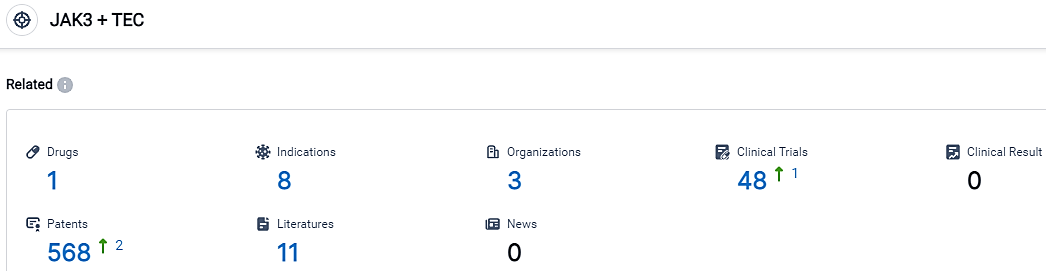
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 20, 2023, there are 1 investigational drugs for the JAK3 and TEC target, including 8 indications,3 R&D institutions involved, with related clinical trials reaching 48,and as many as 568 patents.
LITFULO is a first-of-its-kind treatment which irreversibly and selectively inhibits JAK3 and the TEC family of kinases by blocking γ-common cytokine signaling and reducing cytolytic activity of NK and CD8+ cells. This decreases the activity of parts of the immune system that are involved in the inflammation of hair follicles that causes hair loss in people with alopecia areata.