Peramivir Unveiled: A Detailed Overview of its Revolutionary R&D Breakthroughs
Peramivir's R&D Progress
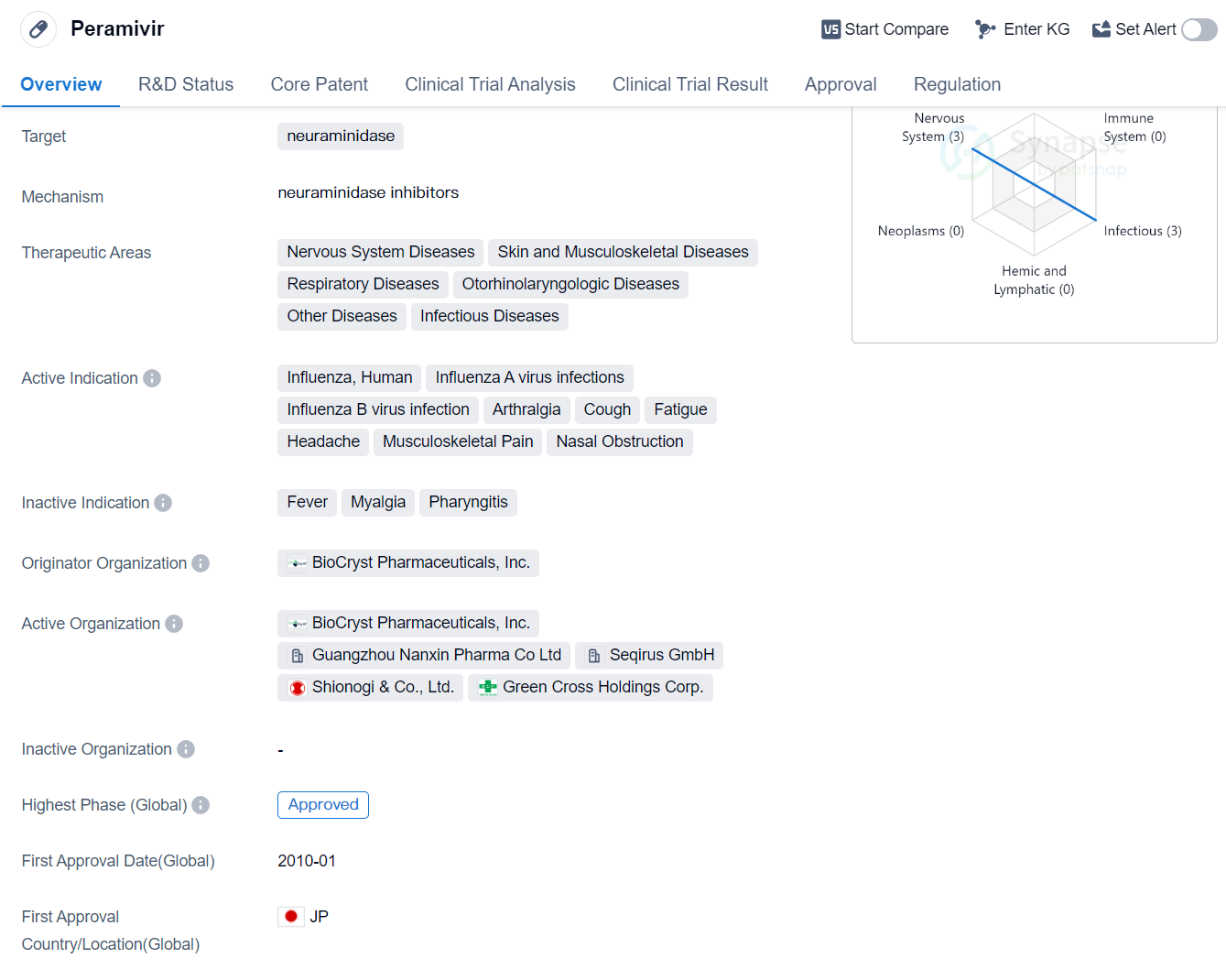
Peramivir is a small molecule drug that targets neuraminidase, an enzyme found on the surface of influenza viruses. It is primarily used for the treatment of various respiratory diseases, including influenza caused by both Influenza A and Influenza B viruses. Additionally, it is indicated for the management of symptoms such as arthralgia, cough, fatigue, headache, musculoskeletal pain, and nasal obstruction.
The drug was developed by BioCryst Pharmaceuticals, Inc., a pharmaceutical company specializing in the discovery and development of small molecule drugs. Peramivir received its first approval in Japan in January 2010, making it available for use in the treatment of influenza in that country. It has also obtained approvals in other countries, although specific details regarding these approvals are not provided.
Peramivir has reached the highest phase of development which is approved globally.
One notable aspect of Peramivir is its regulatory designation as a Fast Track drug. Fast Track is a regulatory process designed to expedite the development and review of drugs that address unmet medical needs. This designation implies that Peramivir may have demonstrated promising results in early clinical trials or has the potential to provide significant benefits over existing treatment options.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Peramivir: neuraminidase inhibitor
Neuraminidase inhibitors are a type of antiviral medication that specifically target the neuraminidase enzyme. Neuraminidase is an enzyme found on the surface of influenza viruses, and it plays a crucial role in the release of newly formed viral particles from infected cells. By inhibiting the activity of neuraminidase, these inhibitors prevent the spread of the virus to other cells in the body.
From a biomedical perspective, neuraminidase inhibitors are commonly used in the treatment and prevention of influenza infections. They work by blocking the neuraminidase enzyme, which hinders the release of viral particles and reduces the severity and duration of symptoms. These medications can be particularly effective when taken within 48 hours of symptom onset.
Neuraminidase inhibitors, such as oseltamivir (Tamiflu) and zanamivir (Relenza), are often prescribed during flu seasons or in the event of an influenza outbreak. They are available in oral or inhaled forms and are generally well-tolerated. It is important to note that these inhibitors are specific to influenza viruses and do not have activity against other types of respiratory viruses.
Drug Target R&D Trends for Peramivir
The analysis of the current competitive landscape and future development of target neuraminidase reveals several key findings. BioCryst Pharmaceuticals, Inc., GSK Plc, and Roche Holding AG are among the companies growing fastest under this target. The approved indications for drugs targeting neuraminidase include Influenza, Human, Influenza A virus infections, and Influenza B virus infection. Small molecule drugs are progressing most rapidly, with small molecule-drug conjugates and prophylactic vaccines also showing potential. Japan, the United States, and China are the countries developing fastest under this target, with significant progress in China's pharmaceutical industry.
Overall, the analysis suggests a competitive landscape with multiple companies and drug types focusing on neuraminidase inhibitors for the treatment of influenza and related indications. The future development of target neuraminidase is expected to continue with advancements in research and potential approvals for additional indications.
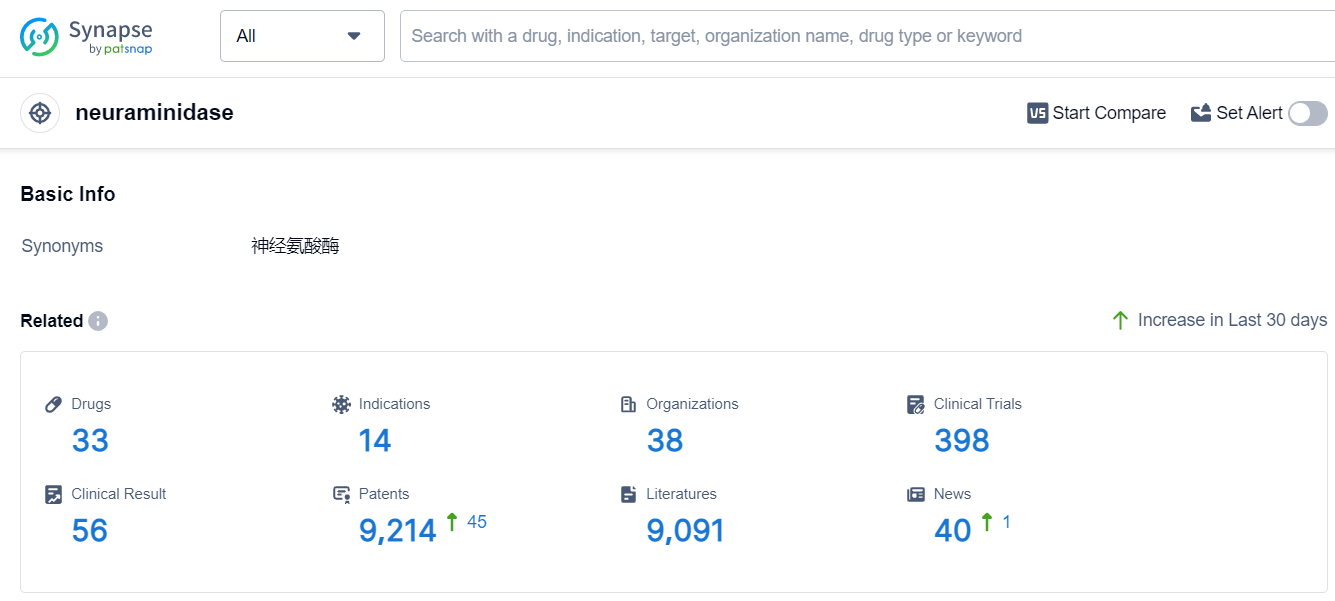
According to Patsnap Synapse, as of 9 Sep 2023, there are a total of 33 neuraminidase drugs worldwide, from 38 organizations, covering 14 indications, and conducting 398 clinical trials.
Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Peramivir is a small molecule drug developed by BioCryst Pharmaceuticals, Inc. It targets neuraminidase and is primarily used for the treatment of respiratory diseases, particularly influenza caused by Influenza A and Influenza B viruses. The drug has received approvals in multiple countries, with its first approval granted in Japan in 2010. Peramivir has reached the highest phase of development, indicating its safety and efficacy, and it has been designated as a Fast Track drug, suggesting its potential to address unmet medical needs.