A Comprehensive Review of Pacritinib's R&D Innovations and Drug Target Mechanism
Pacritinib's R&D Progress
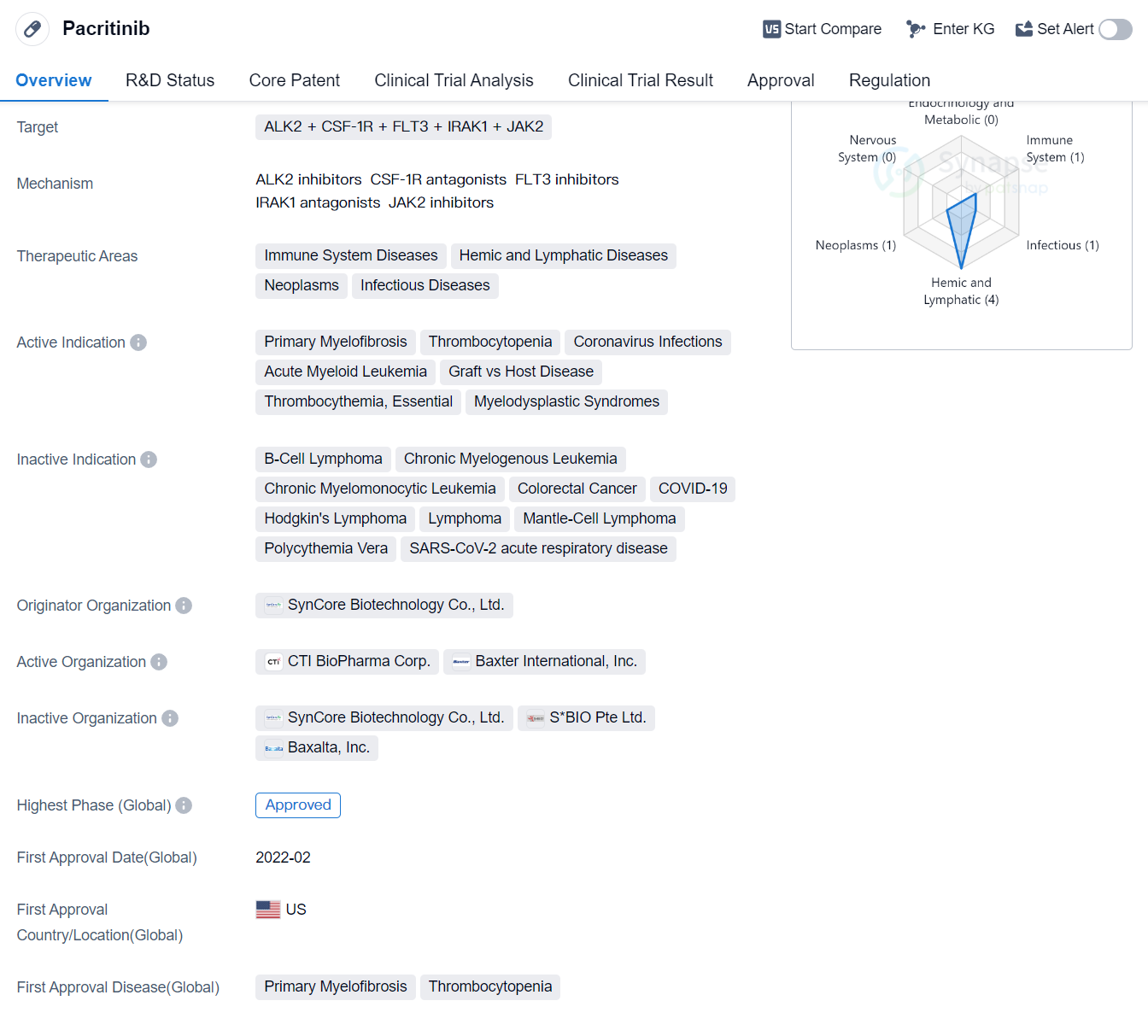
Pacritinib is a small molecule drug that targets multiple proteins including ALK2, CSF-1R, FLT3, IRAK1, and JAK2. It is primarily used in the treatment of immune system diseases, hemic and lymphatic diseases, neoplasms, and infectious diseases. The drug has shown efficacy in treating various conditions such as primary myelofibrosis, thrombocytopenia, coronavirus infections, acute myeloid leukemia, graft vs host disease, thrombocythemia, essential, and myelodysplastic syndromes.
The drug was developed by SynCore Biotechnology Co., Ltd., an originator organization in the pharmaceutical industry. It has reached the highest phase of development, which is approved. The first approval of Pacritinib was granted in the United States in February 2022. The drug has undergone various regulatory processes including priority review, accelerated approval, fast track, and orphan drug designation.
Pacritinib's approval marks a significant milestone in the field of biomedicine, as it offers potential therapeutic options for patients suffering from a range of diseases. Its multi-target approach, targeting proteins involved in various disease pathways, makes it a promising candidate for the treatment of immune system disorders, blood-related diseases, and certain types of cancer.
The drug's approval in the United States suggests that it has met the necessary safety and efficacy requirements set by regulatory authorities. This approval may pave the way for further global approvals and potentially expand the availability of Pacritinib to patients worldwide.
Given the information provided, it is evident that Pacritinib has undergone rigorous testing and evaluation to establish its efficacy and safety profile. The drug's approval in multiple therapeutic areas and indications highlights its potential to address unmet medical needs and improve patient outcomes.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Pacritinib: ALK2 inhibitor and CSF-1R antagonist and FLT3 inhibitor and IRAK1 antagonist and JAK2 inhibitor
ALK2 inhibitors are a type of drugs that specifically target and inhibit the activity of ALK2, which is a receptor involved in various biological processes. From a biomedical perspective, ALK2 is a member of the ALK (Activin receptor-like kinase) family of receptors, which are involved in signaling pathways that regulate cell growth, differentiation, and development. ALK2 inhibitors can be used in the treatment of certain diseases or conditions where ALK2 signaling is dysregulated, such as certain types of cancer or bone disorders.
CSF-1R antagonists are drugs that block the activity of the Colony-Stimulating Factor 1 Receptor (CSF-1R). CSF-1R is a receptor that plays a crucial role in the regulation of the growth and development of macrophages, a type of immune cell. By inhibiting CSF-1R, these antagonists can modulate the immune response and have potential applications in the treatment of diseases involving abnormal macrophage function, such as certain cancers and inflammatory disorders.
FLT3 inhibitors are a class of drugs that target and inhibit the activity of the FLT3 receptor, also known as Fms-like tyrosine kinase 3. FLT3 is a receptor found on the surface of certain blood cells, including hematopoietic stem cells and early progenitor cells. Inhibition of FLT3 can be beneficial in the treatment of certain types of leukemia, where FLT3 mutations are commonly found and contribute to the uncontrolled growth of cancer cells.
IRAK1 antagonists are compounds that inhibit the activity of Interleukin-1 Receptor-Associated Kinase 1 (IRAK1). IRAK1 is an enzyme involved in the signaling pathway of the immune system, specifically in the response to Interleukin-1 (IL-1) cytokines. By blocking IRAK1, these antagonists can modulate the immune response and have potential applications in the treatment of inflammatory diseases and autoimmune disorders.
JAK2 inhibitors are drugs that target and inhibit the activity of Janus Kinase 2 (JAK2), an enzyme involved in the signaling pathway of cytokines and growth factors. JAK2 plays a crucial role in the regulation of hematopoiesis (formation of blood cells) and immune responses. Inhibition of JAK2 can be beneficial in the treatment of certain blood disorders, such as myeloproliferative neoplasms, where JAK2 mutations contribute to abnormal cell growth and differentiation.
In summary, the text refers to different types of drugs that target specific receptors or enzymes involved in various biological processes. These drugs have potential applications in the treatment of diseases or conditions where the targeted molecules are dysregulated or play a significant role.
Drug Target R&D Trends for Pacritinib
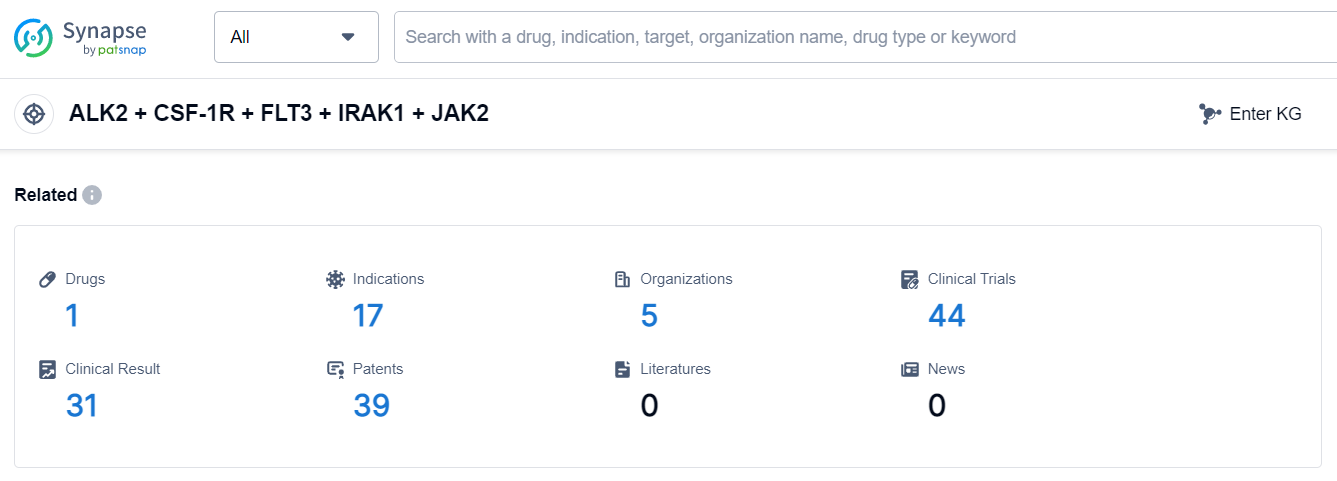
The analysis of the target ALK2 + CSF-1R + FLT3 + IRAK1 + JAK2 reveals that Swedish Orphan Biovitrum AB is the leading company in terms of growth, with drugs in various stages of development. Drugs targeting this target have been approved for indications such as Primary Myelofibrosis and Thrombocytopenia. Small molecule drugs are progressing rapidly, indicating potential competition in the market. The development of drugs under this target is happening globally, with significant progress in countries like the United States, South Korea, Australia, and various countries in the European Union. Further research is needed to understand the progress in China. Overall, the current competitive landscape of target ALK2 + CSF-1R + FLT3 + IRAK1 + JAK2 is dynamic, with potential for future development and competition in the pharmaceutical industry.
According to Patsnap Synapse, as of 6 Sep 2023, there are a total of 1 ALK2 + CSF-1R + FLT3 + IRAK1 + JAK2 drugs worldwide, from 5 organizations, covering 17 indications, and conducting 44 clinical trials.
Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In conclusion, Pacritinib is a small molecule drug developed by SynCore Biotechnology Co., Ltd. It targets multiple proteins and has been approved for use in the treatment of various immune system diseases, hemic and lymphatic diseases, neoplasms, and infectious diseases. Its approval in the United States and its designation as a priority review, accelerated approval, fast track, and orphan drug indicate its potential as a therapeutic option for patients. Further research and development may explore its efficacy in additional indications and expand its availability globally.