Pharmaceutical Insights: Nitrofurazone's R&D Progress and its Mechanism of Action on Drug Target
Nitrofurazone's R&D Progress
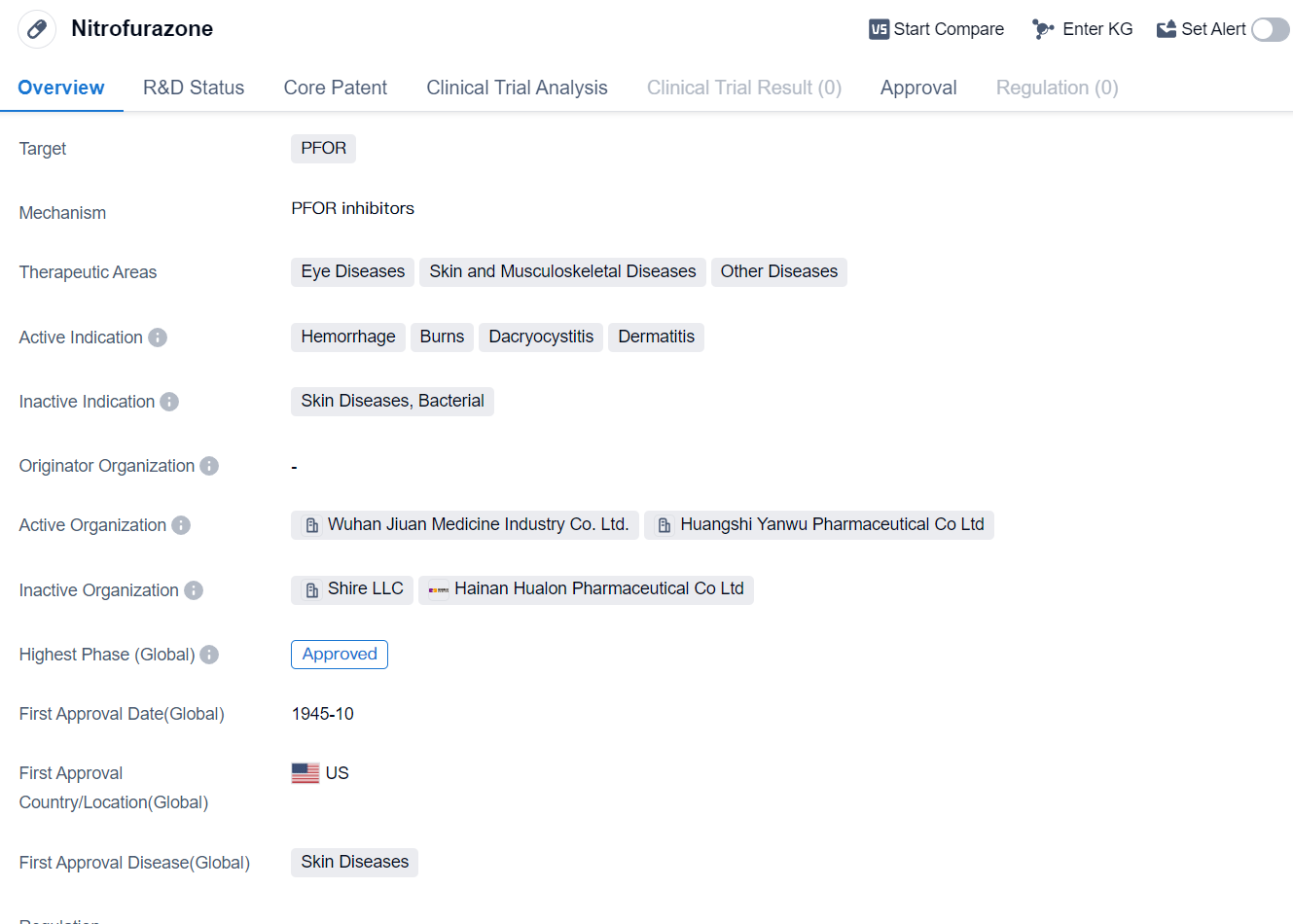
Nitrofurazone is a small molecule drug that is primarily used in the treatment of various eye diseases, skin and musculoskeletal diseases, and other diseases. It is indicated for the management of conditions such as hemorrhage, burns, dacryocystitis, and dermatitis.
The drug targets PFOR, which stands for pyruvate:ferredoxin oxidoreductase. PFOR is an enzyme that plays a crucial role in the metabolism of microorganisms, making it an attractive target for drug development.
Nitrofurazone has a long history in the pharmaceutical industry, with its first approval dating back to October 1945 in the United States. It is worth noting that the drug has received approvals in the global market , indicating its widespread use and recognition.
The highest R&D phase of this drug is approved. It suggests that the drug has been thoroughly evaluated and deemed suitable for use in patients.
The therapeutic areas in which Nitrofurazone is indicated highlight its versatility and potential for treating a range of conditions. Eye diseases, such as dacryocystitis, involve inflammation or infection of the tear ducts, while skin and musculoskeletal diseases encompass a wide range of conditions affecting the skin, muscles, and bones. Additionally, Nitrofurazone is indicated for other diseases, indicating its potential for addressing various medical conditions beyond the specified therapeutic areas.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Nitrofurazone: PFOR inhibitor
PFOR inhibitors are a type of chemical compounds or drugs that target and inhibit the activity of the enzyme pyruvate:ferredoxin oxidoreductase (PFOR). PFOR is an important enzyme involved in the metabolism of certain microorganisms, particularly anaerobic bacteria and archaea.
From a biomedical perspective, PFOR inhibitors can be used as potential therapeutic agents to treat infections caused by anaerobic bacteria. By inhibiting the activity of PFOR, these inhibitors disrupt the energy production and metabolism of the bacteria, ultimately leading to their growth inhibition or death.
PFOR inhibitors have shown promise in the treatment of various infections, including those caused by Clostridium difficile, a bacterium responsible for severe gastrointestinal infections. Inhibiting PFOR can weaken the bacterium's ability to survive and replicate, making it more susceptible to other antimicrobial treatments.
Further research and development of PFOR inhibitors may contribute to the development of new antibiotics or antimicrobial agents that can effectively target anaerobic bacteria and improve treatment options for related infections.
Drug Target R&D Trends for Nitrofurazone
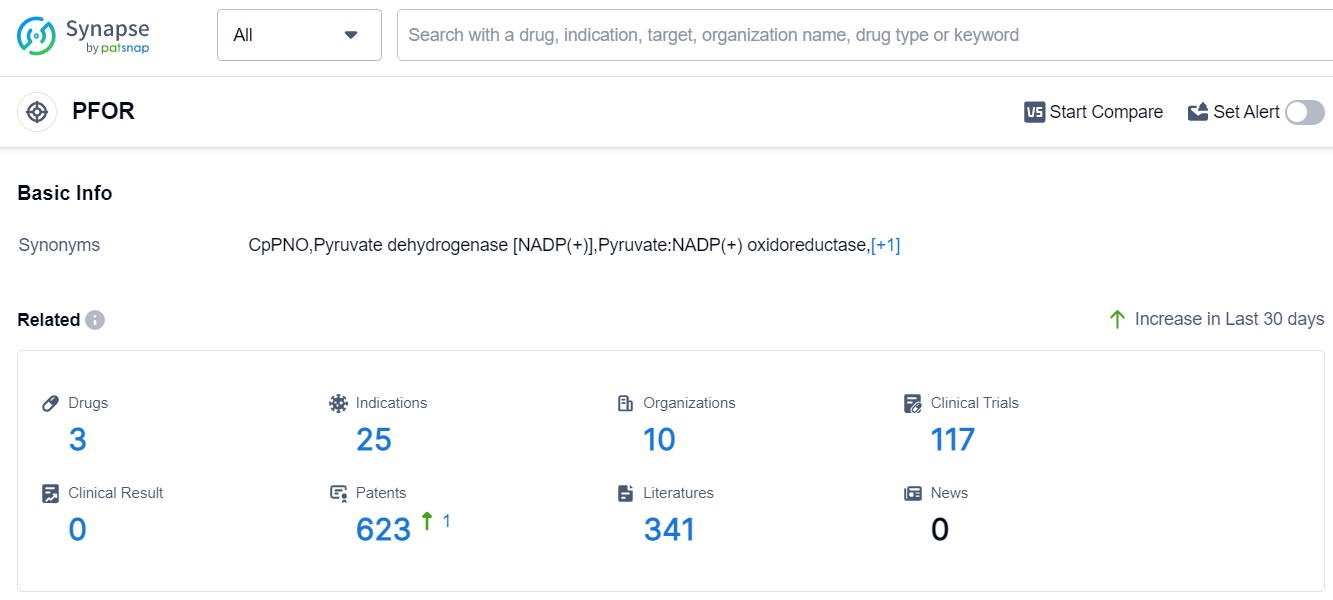
The current competitive landscape of the target PFOR is characterized by the presence of multiple companies with drugs in various stages of development. Romark LC, Huangshi Yanwu Pharmaceutical Co Ltd, and Hubei Jiu'an Pharmaceutical Group Co., Ltd. are the fastest-growing companies under this target. The approved drugs cover a wide range of indications, indicating a diverse treatment portfolio. Small molecule drugs are progressing rapidly, with several approved and inactive drugs. China and the United States are the leading countries in terms of drug development, with China showing significant progress. Overall, the target PFOR has a promising competitive landscape and future development potential.
According to Patsnap Synapse, as of 8 Sep 2023, there are a total of 3 PFOR drugs worldwide, from 10 organizations, covering 25 indications, and conducting 117 clinical trials.
Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Nitrofurazone is a small molecule drug that targets PFOR and is approved for use in the treatment of eye diseases, skin and musculoskeletal diseases, and other diseases. Its long history, global approvals, and highest phase of development highlight its significance in the pharmaceutical industry. However, further research and analysis may be required to fully understand its mechanism of action, efficacy, and potential side effects.