Pfizer Reveals Promising Phase 3 Results for Hemophilia A Gene Therapy Candidate
Pfizer Inc. revealed encouraging topline outcomes from the Phase 3 AFFINE trial, which assesses giroctocogene fitelparvovec. This experimental gene therapy aims to treat adult patients suffering from moderately severe to severe hemophilia A.
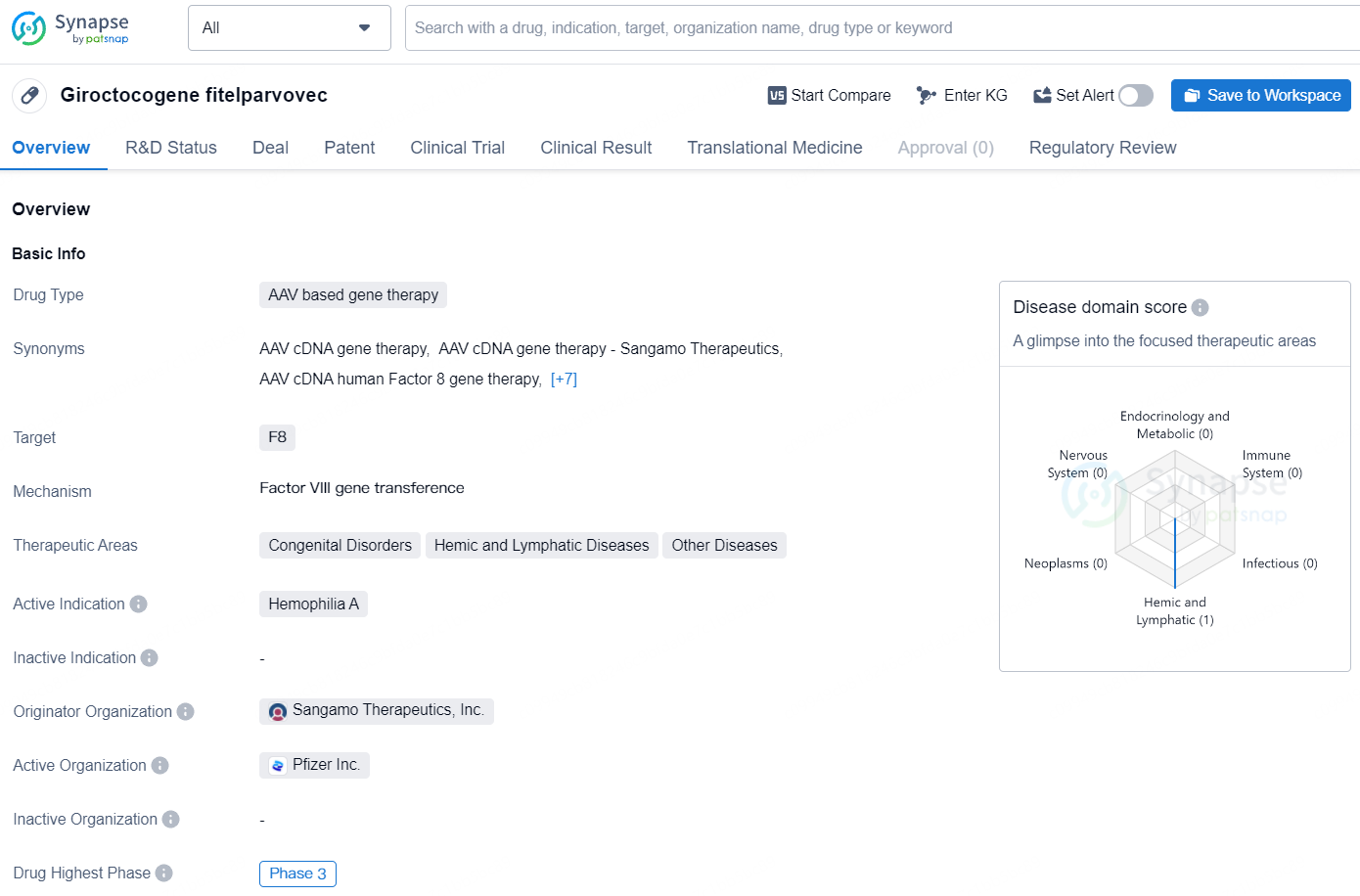
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The AFFINE trial successfully met its primary goal of establishing non-inferiority, and also surpassed it, in terms of the total annualized bleeding rate from Week 12 through a minimum of 15 months post-infusion compared to standard Factor VIII (FVIII) replacement prophylaxis therapy. A single dose of 3e13 vg/kg of giroctocogene fitelparvovec resulted in a statistically significant reduction in mean total ABR when contrasted with the pre-infusion period.
The key secondary endpoints, as set by the trial's protocol, were achieved and further showed superiority over prophylaxis. At 15 months post-infusion, 84% of participants maintained FVIII activity levels exceeding 5%, with the majority recording FVIII activity of at least 15%. The mean treated ABR exhibited a statistically significant decrease of 98.3%, dropping from 4.08 pre-infusion to 0.07 post-infusion. Throughout the study, only one participant returned to prophylaxis after infusion.
In the AFFINE study, giroctocogene fitelparvovec was generally well tolerated. Temporary elevated FVIII levels of ≥150% were documented in 49.3% of dosed participants via chromogenic assays, without affecting the efficacy and safety outcomes. Serious adverse events were recorded in 15 patients, with 13 events reported by 10 patients being attributed to treatment. These treatment-related adverse events were typically resolved with clinical management.
"We are extremely satisfied with the positive outcomes from the Phase 3 AFFINE study, which demonstrate the safety and efficacy of our one-time gene therapy candidate for patients with hemophilia A," stated James Rusnak, M.D., Ph.D., Senior Vice President, Chief Development Officer, Internal Medicine and Infectious Diseases, Research and Development, Pfizer. "We are eager to progress this innovative solution to alleviate the medical and treatment burdens associated with frequent and time-consuming intravenous infusions or injections, continuing Pfizer's more than four-decade-long commitment to advancing hemophilia treatment."
Pfizer has recently received FDA approval for its hemophilia B gene therapy, BEQVEZ™ (fidanocogene elaparvovec). BEQVEZ has also been approved in Canada and is currently pending a decision from the European Medicines Agency following a favorable opinion from the EMA’s Committee for Medicinal Products for Human Use in May 2024.
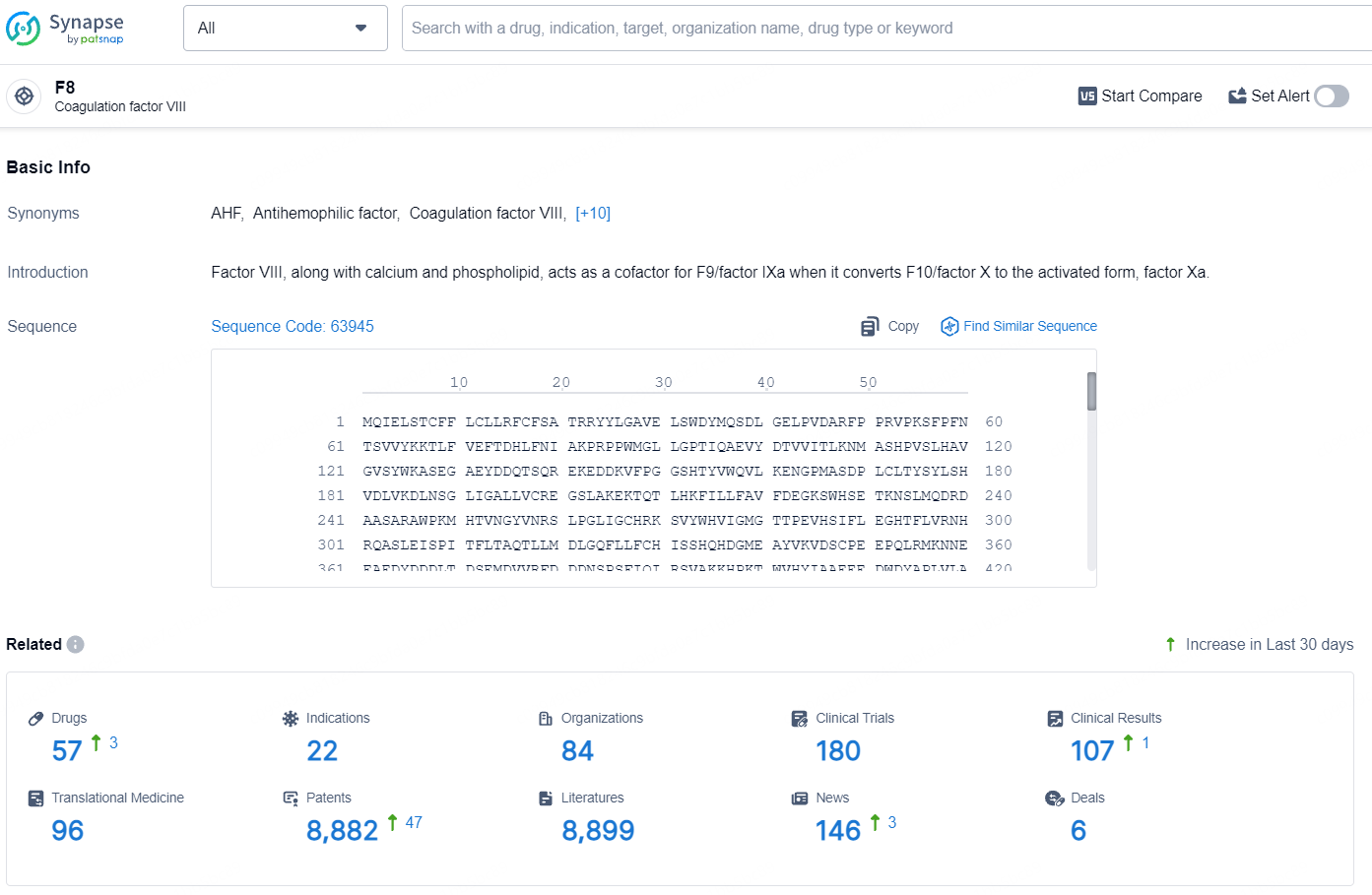
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 30, 2024, there are 57 investigational drugs for the FVIII target, including 22 indications, 84R&D institutions involved, with related clinical trials reaching 180, and as many as 8882 patents.
Giroctocogene fitelparvovec is a novel, investigational gene therapy that contains a bio-engineered AAV6 capsid and a modified B-domain deleted human coagulation FVIII gene. The goal of this investigational treatment for people living with hemophilia A is that a single infusion of giroctocogene fitelparvovec may allow them to produce FVIII themselves for an extended period of time, providing bleed protection and reducing the need for routine prophylaxis with intravenous infusions or injections.