Is Albuterol/Budesonide approved by the FDA?
The FDA approved the combination of albuterol and budesonide under the brand name Airsupra on January 10, 2023. This approval provides a new option for adults needing relief from asthma symptoms and prevention of asthma attacks.
Uses for Albuterol/Budesonide
Albuterol and budesonide inhalation is indicated for the treatment or prevention of bronchoconstriction and to reduce the risk of exacerbations in adult patients with asthma. This combination is intended for occasional use and not as a maintenance treatment for asthma.
Administration and Dosage
The typical adult dose for asthma is two actuations of albuterol 90 mcg and budesonide 80 mcg as needed, with a maximum of 6 doses (12 inhalations) per day. The inhaler should be shaken well before each use. It is important to prime the inhaler before the first use by releasing 4 sprays into the air, away from the face. Re-prime with 2 sprays if the inhaler has not been used for more than 7 days, after cleaning, or if it has been dropped. Users should rinse their mouth with water after each use to reduce the risk of fungal infections in the mouth or throat.
Precautions and Side Effects
Warnings:
- Use only as directed. Inform your doctor if you use other medicines or have other medical conditions or allergies.
- Long-term use of steroid medicine can lead to weak bones (osteoporosis).
- It is not known if albuterol and budesonide inhalation will harm an unborn baby. Having asthma during pregnancy may increase the risk of premature birth or low birth weight. Discuss the benefits and risks with your doctor.
Common Side Effects:
- Sores or white patches in the mouth or throat
- Trouble swallowing
- Headache
- Cough
- Hoarse or deepened voice
Serious Side Effects:
- Heart problems, high blood pressure, or fast heart rate
- Low blood potassium, leading to symptoms like leg cramps, constipation, irregular heartbeats, or muscle weakness
- Decreased adrenal gland hormones, causing nausea, vomiting, stomach pain, loss of appetite, or skin discoloration
- Signs of infection, such as fever, chills, sore throat, body aches, unusual tiredness, or bruising
- Eye problems, including glaucoma and cataracts
- Bone problems, such as bone pain
Patients should seek emergency medical help if they experience signs of an allergic reaction, such as hives, difficulty breathing, or swelling of the face, lips, tongue, or throat.
Conclusion
Albuterol and budesonide, under the brand name Airsupra, was approved by the FDA on January 10, 2023, for the treatment and prevention of asthma symptoms in adults. This combination inhalation medication offers a new option for managing asthma symptoms effectively. It is essential for patients to follow their healthcare provider's instructions and be aware of the potential side effects and necessary precautions when using this medication.
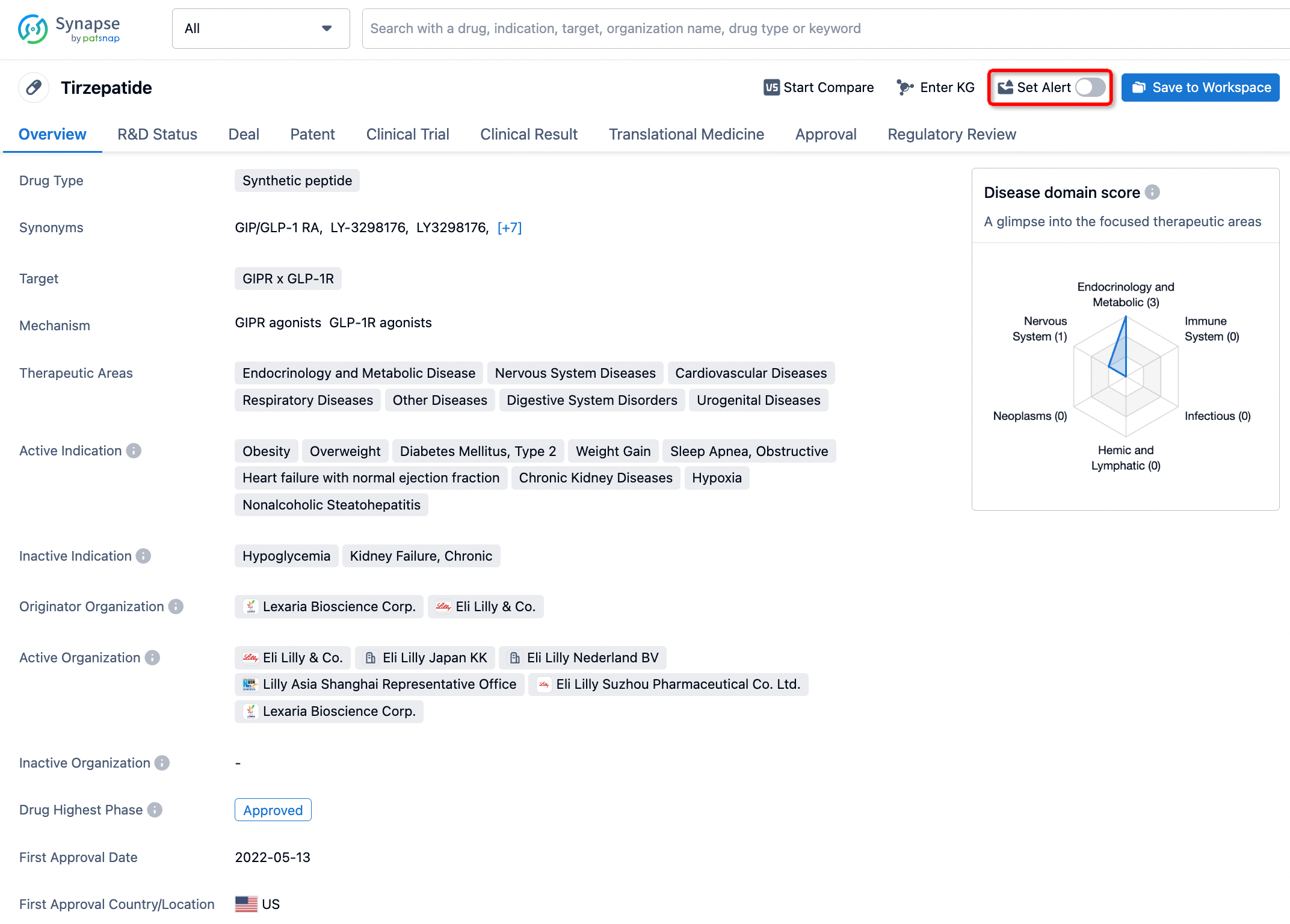
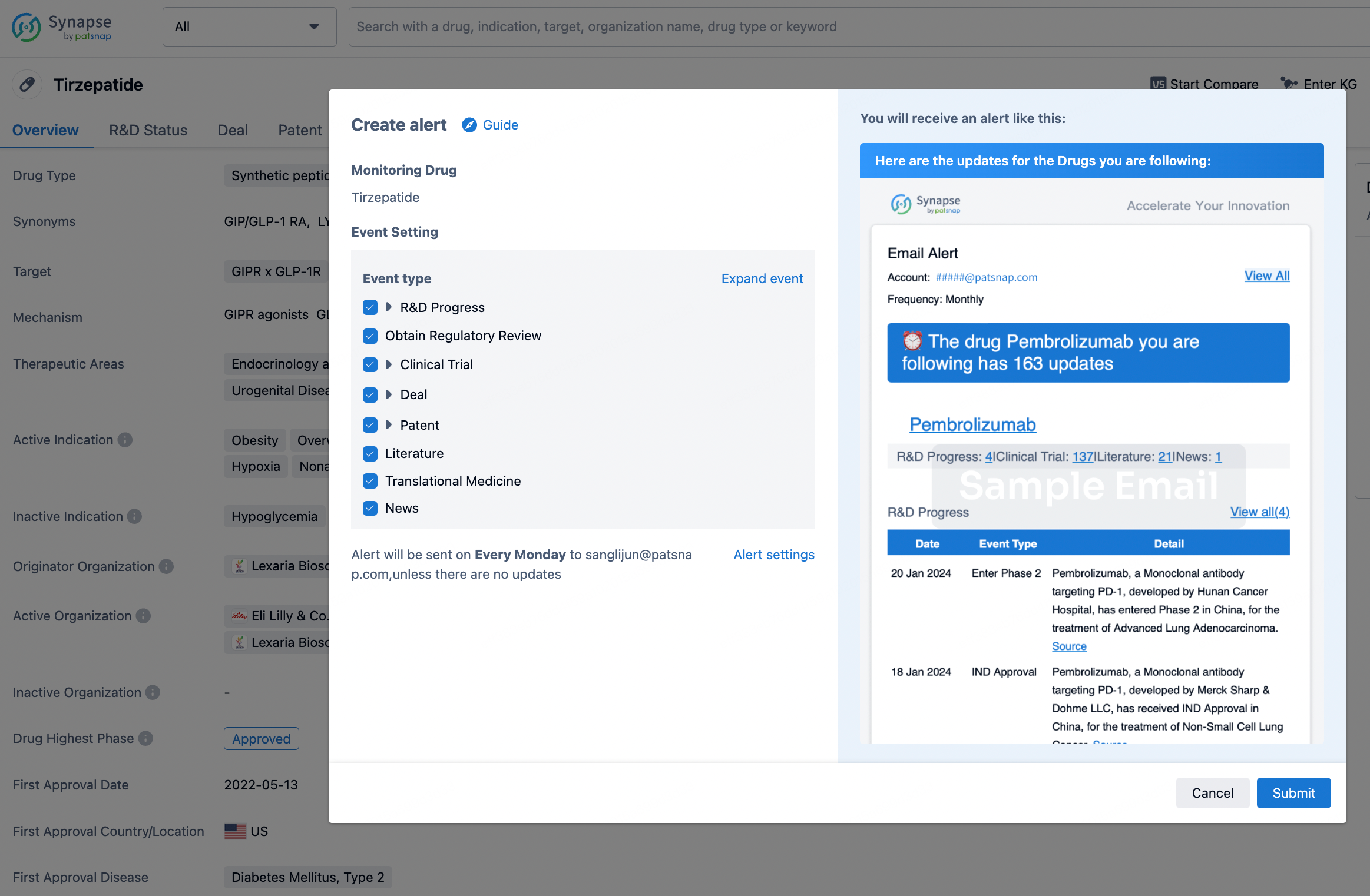
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!