Pharmaceutical Insights: Clevidipine's R&D Progress and its Mechanism of Action on Drug Target
Clevidipine's R&D Progress
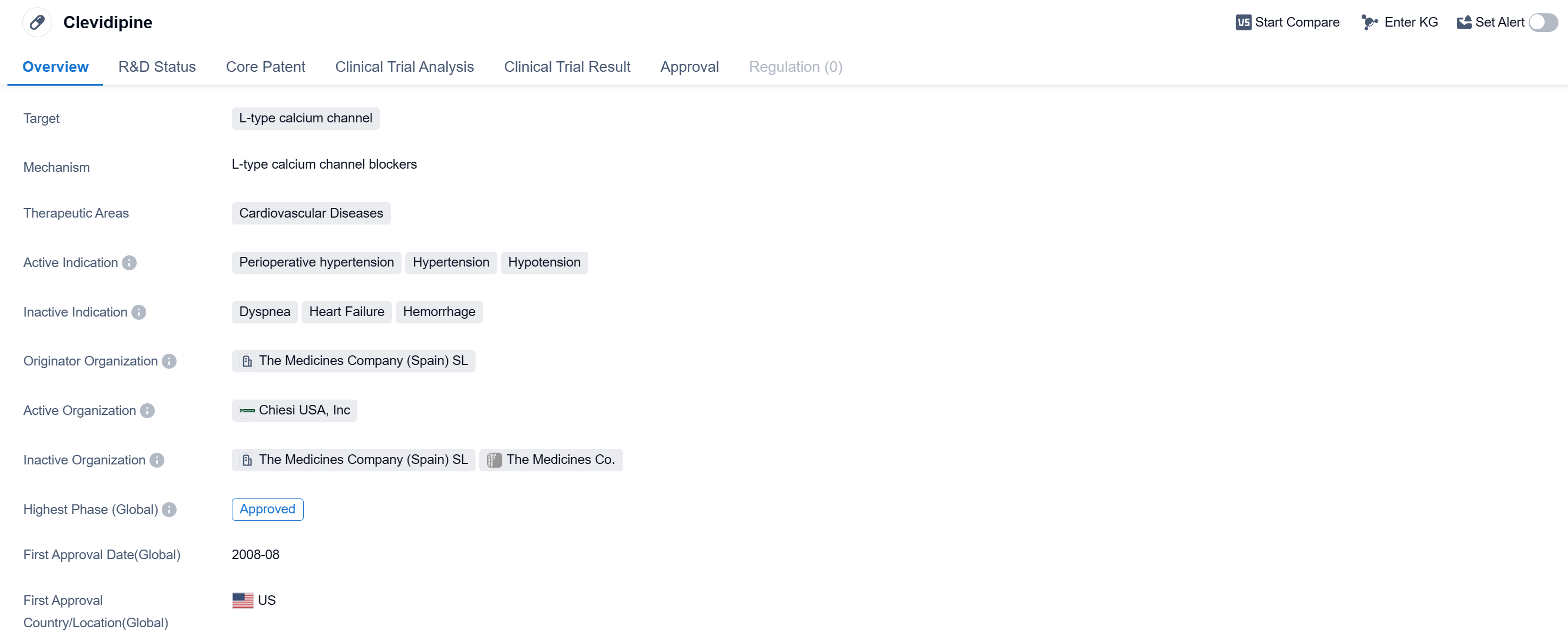
Clevidipine is a small molecule drug that belongs to the therapeutic area of cardiovascular diseases. It specifically targets the L-type calcium channel, which plays a crucial role in regulating blood pressure. The drug has been approved for use in the treatment of perioperative hypertension, hypertension, and hypotension.
The originator organization of Clevidipine is The Medicines Company (Spain) SL. The drug has reached the highest phase of development, which is the approved stage.
Clevidipine received its first approval in the United States in August 2008. This suggests that it has been available in the market for over a decade and has gained recognition and acceptance by regulatory authorities.
As a small molecule drug, Clevidipine is designed to interact with the L-type calcium channel, which is involved in the regulation of calcium ions in smooth muscle cells. By blocking this channel, Clevidipine helps to relax and dilate blood vessels, leading to a reduction in blood pressure. This mechanism of action makes it particularly suitable for the treatment of cardiovascular conditions such as hypertension and hypotension.
The approval of Clevidipine for perioperative hypertension indicates its potential to effectively manage high blood pressure during surgical procedures. This is crucial as perioperative hypertension can lead to complications and adverse outcomes for patients undergoing surgery.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Clevidipine: L-type calcium channel blockers
L-type calcium channel blockers are a type of medication that is used to treat various conditions, particularly cardiovascular disorders. From a biomedical perspective, these blockers specifically target L-type calcium channels, which are found in the heart and smooth muscle cells. These channels play a crucial role in regulating the influx of calcium ions into cells, which is necessary for muscle contraction.
By blocking these channels, L-type calcium channel blockers reduce the entry of calcium ions into cardiac and smooth muscle cells. This leads to relaxation and dilation of blood vessels, decreased cardiac contractility, and a reduction in heart rate. As a result, these medications are effective in treating conditions such as hypertension (high blood pressure), angina (chest pain), and certain arrhythmias (abnormal heart rhythms).
L-type calcium channel blockers are commonly prescribed drugs and are available in various forms, including oral tablets and extended-release formulations. They are often used as a first-line treatment for hypertension and angina, and can also be combined with other medications to achieve optimal control of cardiovascular conditions.
It's important to note that L-type calcium channel blockers may have side effects, such as dizziness, flushing, constipation, and ankle swelling. Therefore, it is essential to consult with a healthcare professional before starting or adjusting the dosage of these medications.
Drug Target R&D Trends for Clevidipine
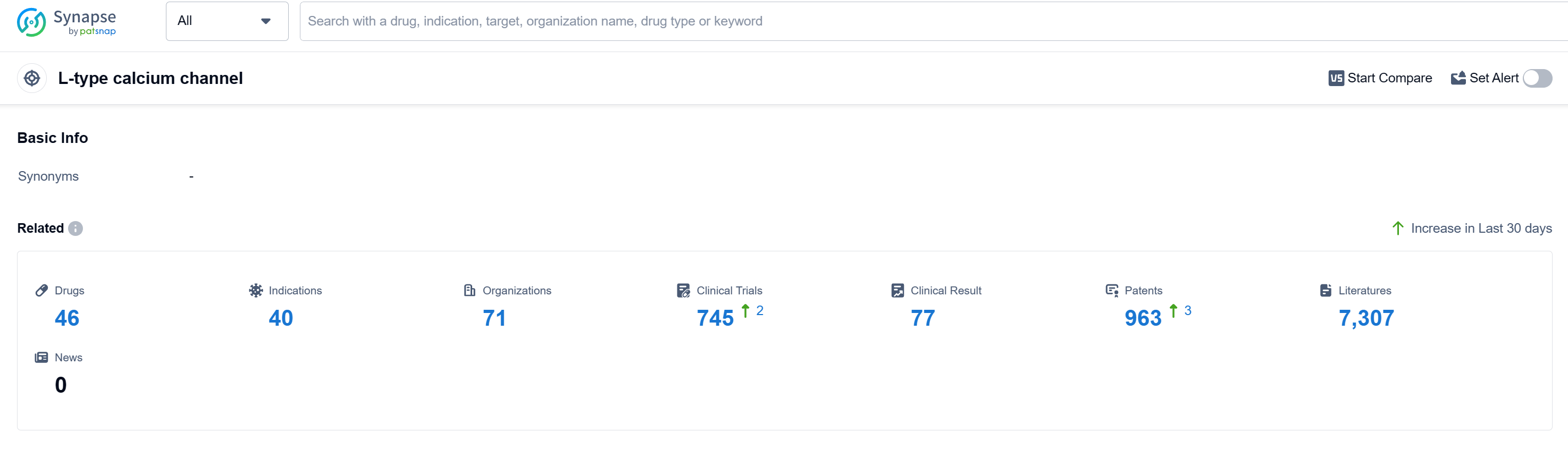
According to Patsnap Synapse, as of 16 Sep 2023, there are a total of 46 L-type calcium channel drugs worldwide, from 71 organizations, covering 40 indications, and conducting 745 clinical trials.
The analysis of the target L-type calcium channel reveals a competitive landscape with multiple companies actively involved in research and development. Daiichi Sankyo Co., Ltd., Viatris Inc., CSPC Pharmaceutical Group Ltd., and Chong Kun Dang Pharmaceutical Corp. are some of the companies growing fastest under this target. The highest stage of development is the "Approved" phase, with several companies having drugs in this phase. Hypertension is the most common approved indication, and small molecule drugs are progressing rapidly. Japan, China, and the United States are the leading countries in terms of drug development under this target. The progress in China is particularly noteworthy, with several drugs in the "Approved" phase. Overall, the target L-type calcium channel presents a competitive landscape with ongoing research and development efforts, indicating potential future advancements in the field.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Clevidipine is a small molecule drug that targets the L-type calcium channel and is approved for the treatment of perioperative hypertension, hypertension, and hypotension. Its approval in the United States in 2008 highlights its established presence in the market. With its mechanism of action, Clevidipine offers a promising option for managing cardiovascular diseases and ensuring optimal blood pressure control in various clinical settings.