Deep Scientific Insights on Cangrelor's R&D Progress, Mechanism of Action, and Drug Target
Cangrelor's R&D Progress
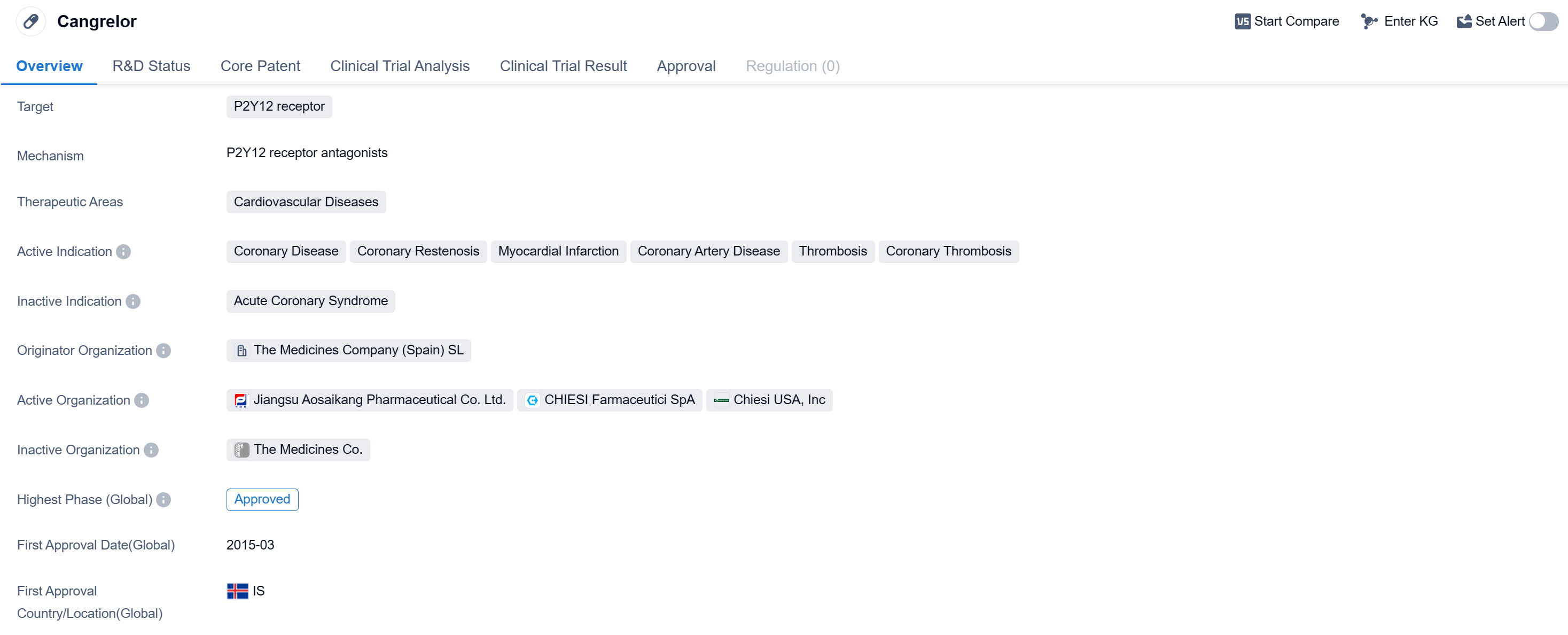
Cangrelor is a small molecule drug that targets the P2Y12 receptor and is primarily used in the treatment of cardiovascular diseases. It has been approved for use in various indications related to coronary disease, including coronary restenosis, myocardial infarction, coronary artery disease, thrombosis, and coronary thrombosis. The drug was first approved in Iceland in March 2015.
Cangrelor is developed by The Medicines Company (Spain) SL, an originator organization in the pharmaceutical industry. It is important to note that while the drug has reached the highest phase of approval globally, it is still in Phase 1 in China, indicating ongoing development and potential future approvals in that market.
The drug's mechanism of action involves targeting the P2Y12 receptor, which is involved in platelet activation and aggregation. By inhibiting this receptor, Cangrelor helps prevent the formation of blood clots, which are a common complication in cardiovascular diseases.
As a small molecule drug, it is likely to have a well-defined chemical structure and can be easily synthesized in a laboratory setting. This characteristic may contribute to its potential for widespread use and availability.
Given its therapeutic focus on cardiovascular diseases, Cangrelor may have a significant impact on patient outcomes and the management of these conditions. By targeting the P2Y12 receptor, the drug offers a specific and targeted approach to preventing blood clot formation, which is crucial in reducing the risk of complications such as coronary restenosis, myocardial infarction, and thrombosis.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Cangrelor: P2Y12 receptor antagonists
P2Y12 receptor antagonists are a type of medication that act on the P2Y12 receptors in the body. From a biomedical perspective, P2Y12 receptors are a subtype of purinergic receptors that are primarily found on blood platelets. These receptors play a crucial role in platelet aggregation, which is the process of platelets clumping together to form blood clots. P2Y12 receptor antagonists work by binding to these receptors and inhibiting their activation, thereby preventing platelet aggregation and reducing the risk of blood clot formation.
By blocking the P2Y12 receptors, these antagonists interfere with the signaling pathway that leads to platelet activation and aggregation. This inhibition is particularly important in the context of cardiovascular diseases, such as acute coronary syndrome or stroke, where excessive platelet aggregation can lead to the formation of dangerous blood clots that can block blood vessels and cause tissue damage.
P2Y12 receptor antagonists are commonly used as antiplatelet drugs to prevent clotting in patients with conditions such as coronary artery disease or those who have undergone certain cardiovascular procedures, such as stent placement. Examples of P2Y12 receptor antagonists include clopidogrel, ticagrelor, and prasugrel. These medications are typically prescribed in combination with other antiplatelet drugs or anticoagulants to provide a comprehensive approach to preventing clot formation and reducing the risk of cardiovascular events.
Drug Target R&D Trends for Cangrelor
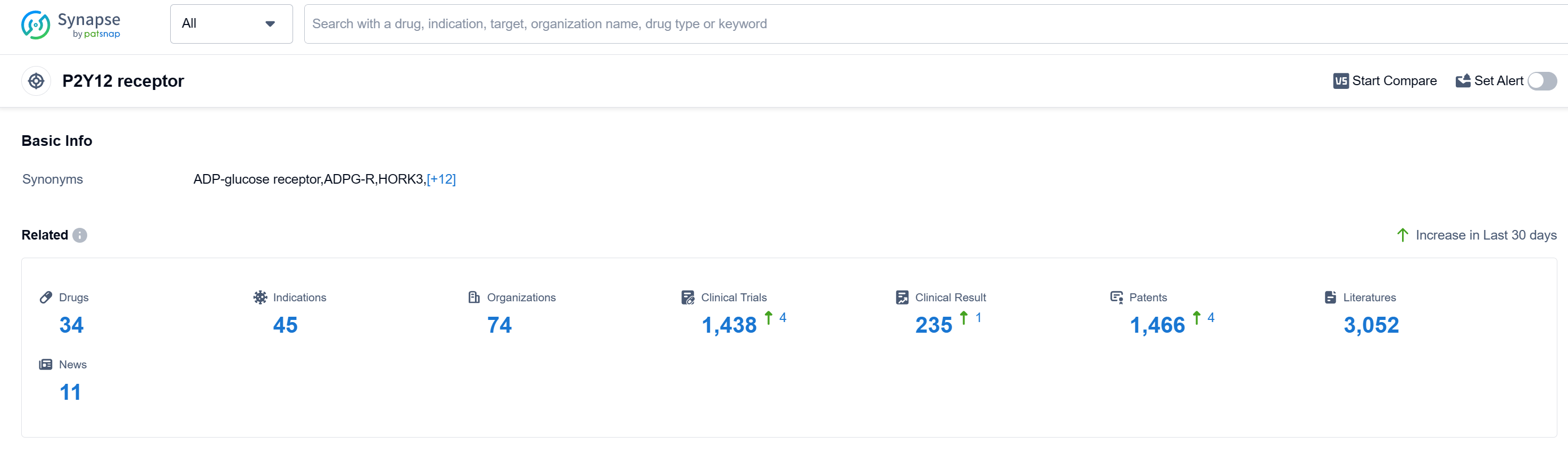
According to Patsnap Synapse, as of 15 Sep 2023, there are a total of 34 P2Y12 receptor drugs worldwide, from 74 organizations, covering 45 indications, and conducting 1438 clinical trials.
The analysis of the target P2Y12 receptor reveals a competitive landscape with multiple companies actively involved in the development of drugs. The highest stage of development is the approved phase, indicating successful clinical trials and potential market availability. The indications for these drugs cover a wide range of cardiovascular diseases and conditions, highlighting the potential therapeutic applications of targeting the P2Y12 receptor. Small molecule drugs are the most rapidly progressing drug type, indicating their effectiveness and suitability for targeting this receptor. China has shown significant progress in the development of drugs targeting the P2Y12 receptor, indicating its growing presence in the pharmaceutical industry. Overall, the target P2Y12 receptor presents a promising opportunity for pharmaceutical companies and researchers in the cardiovascular field.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In conclusion, Cangrelor is a small molecule drug developed by The Medicines Company (Spain) SL, targeting the P2Y12 receptor for the treatment of cardiovascular diseases. With its approval in Iceland and ongoing development in China, the drug shows promise in improving patient outcomes and managing conditions such as coronary disease and thrombosis. Further research and development may lead to additional approvals and expanded use of Cangrelor in the future.