Pharmaceutical Insights: Colestipol Hydrochloride's R&D Progress and its Mechanism of Action on Drug Target
Colestipol Hydrochloride's R&D Progress
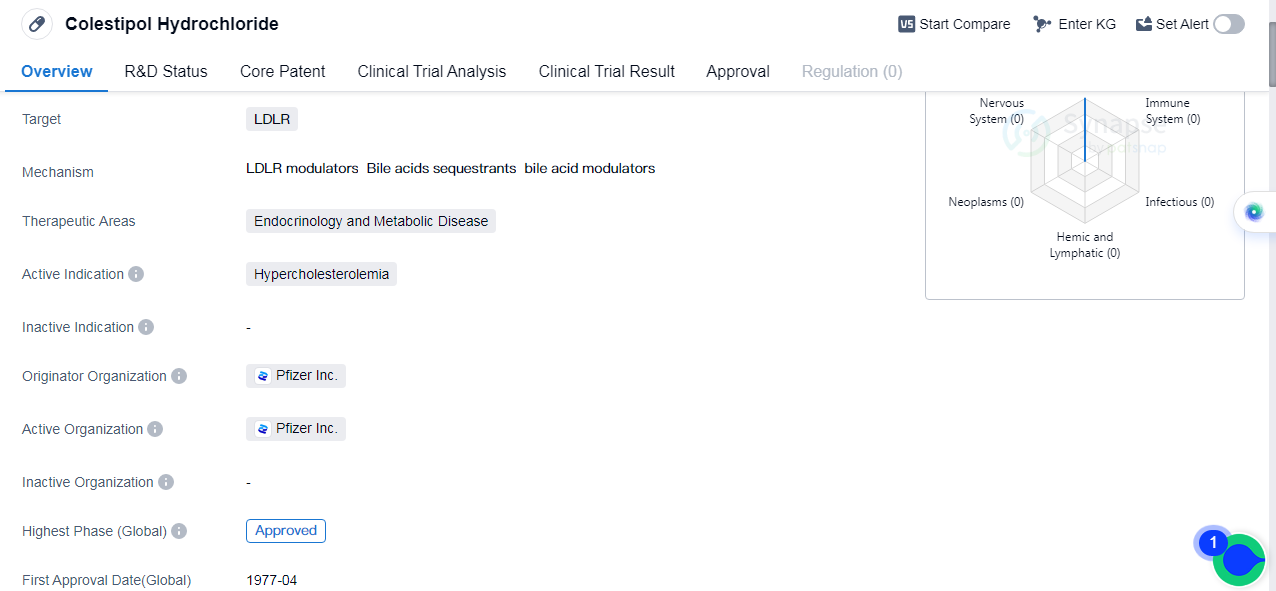
Colestipol Hydrochloride is a small molecule drug that falls under the therapeutic areas of endocrinology and metabolic disease. It is primarily used for the treatment of hypercholesterolemia, a condition characterized by high levels of cholesterol in the blood. The drug targets low-density lipoprotein receptor(LDLR).
Colestipol Hydrochloride was first approved in the United States in April 1977. It is worth noting that this drug has reached the highest phase of development, which is the approved phase. This indicates that it has successfully completed all necessary clinical trials and has been deemed safe and effective for use in patients.
The originator organization of Colestipol Hydrochloride is Pfizer Inc., a renowned pharmaceutical company. As the originator, Pfizer Inc. is responsible for the initial discovery, development, and marketing of the drug.
Colestipol Hydrochloride works by binding to bile acids in the intestine, preventing their reabsorption. This leads to an increased excretion of bile acids, which in turn stimulates the liver to produce more bile acids from cholesterol. As a result, the levels of cholesterol in the blood are reduced, specifically the low-density lipoprotein (LDL) cholesterol, often referred to as bad cholesterol.
Hypercholesterolemia is a significant risk factor for cardiovascular diseases, including heart attacks and strokes. By effectively lowering LDL cholesterol levels, Colestipol Hydrochloride plays a crucial role in managing this condition and reducing the associated cardiovascular risks.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Colestipol Hydrochloride: LDLR modulators, bile acid sequestrants, and bile acid modulators
LDLR modulators, bile acid sequestrants, and bile acid modulators are terms related to biomedicine and are all involved in the regulation of cholesterol metabolism.
LDLR modulators refer to substances or drugs that can modulate the function of the LDL receptor (LDLR). The LDLR is responsible for removing LDL cholesterol from the bloodstream. By modulating the LDLR, these substances can either enhance or inhibit its activity, thereby affecting the clearance of LDL cholesterol.
Bile acids sequestrants are drugs that bind to bile acids in the intestine, preventing their reabsorption. Bile acids are synthesized from cholesterol in the liver and are essential for the digestion and absorption of dietary fats. By sequestering bile acids, these drugs reduce the amount of bile acids available for reabsorption, leading to increased conversion of cholesterol into bile acids and ultimately reducing the levels of LDL cholesterol in the blood.
Bile acid modulators encompass a broader category of drugs that can influence the synthesis, metabolism, or transport of bile acids. They can affect various steps in the bile acid pathway, including the synthesis of bile acids in the liver, their transport to the gallbladder for storage, and their release into the intestine for digestion. By modulating these processes, bile acid modulators can impact cholesterol metabolism and potentially lower LDL cholesterol levels.
Overall, LDLR modulators, bile acid sequestrants, and bile acid modulators are all involved in the regulation of cholesterol levels, with the aim of reducing LDL cholesterol, which is commonly referred to as bad cholesterol. These approaches are often used in the management of hypercholesterolemia and the prevention of cardiovascular diseases.
Drug Target R&D Trends for Colestipol Hydrochloride
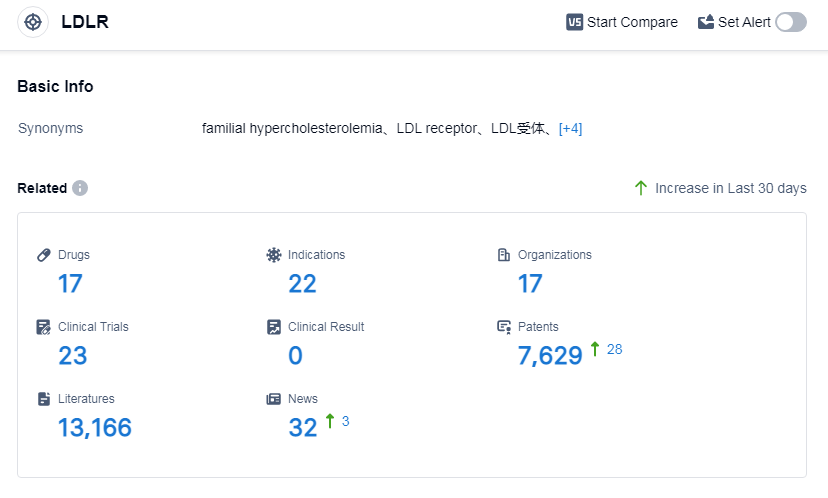
According to Patsnap Synapse, as of 6 Sep 2023, there are a total of 17 LDLR drugs worldwide, from 17 organizations, covering 22 indications, and conducting 23 clinical trials.
Based on the analysis of the target LDLR, it can be concluded that Pfizer Inc. is the leading company in terms of the highest stage of development with an approved drug. The indications being targeted by drugs under this target range from hypercholesterolemia to various types of cancers and other diseases. The drug types progressing most rapidly include small molecule drugs, peptide drug conjugates, and AAV based gene therapy. The countries/locations developing fastest under this target include the United States, Canada, China, and the European Union. The future development of target LDLR is expected to continue with a focus on innovative drugs and intense competition in the biosimilar market.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Colestipol Hydrochloride is a small molecule drug developed by Pfizer Inc. It is approved for the treatment of hypercholesterolemia and works by targeting LDLR. With its ability to lower LDL cholesterol levels, this drug plays a vital role in managing this condition and reducing the associated cardiovascular risks.