Pharmaceutical Insights: Ibudilast's R&D Progress and its Mechanism of Action on Drug Target
Ibudilast's R&D Progress
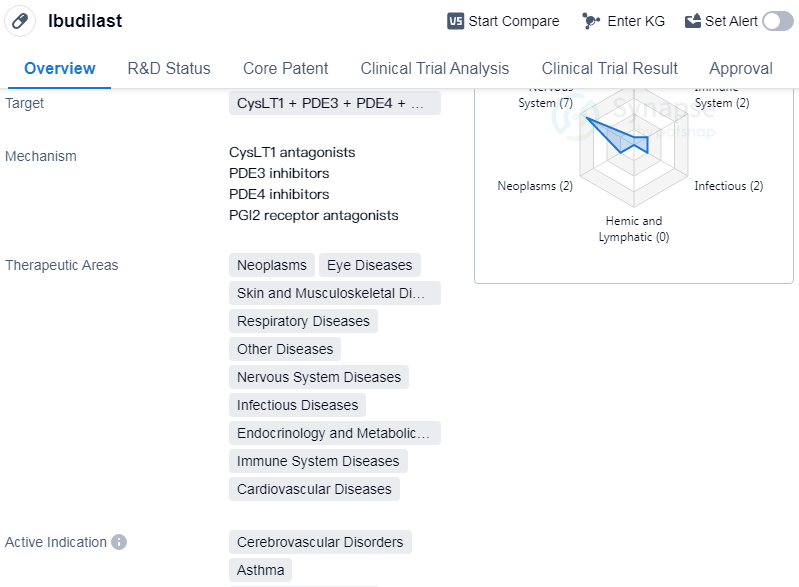
Ibudilastis a small molecule drug that targets multiple receptors including CysLT1, PDE3, PDE4, and the PGI2 receptor. It has a wide range of therapeutic areas including neoplasms, eye diseases, skin and musculoskeletal diseases, respiratory diseases, nervous system diseases, etc.
The drug has been indicated for various conditions such as cerebrovascular disorders, asthma, cerebral hemorrhage, multiple sclerosis, and acute lung injury.
Ibudilast was developed by KYORIN Pharmaceutical Co., Ltd., and received its first approval in Japan in January 1989. It is currently in the highest phase of development, which is approved globally. The drug is regulated as an orphan drug, indicating its potential for treating rare diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Ibudilast: CysLT1 antagonists, PDE3 inhibitors, PDE4 inhibitors and PGI2 receptor antagonists
CysLT1 antagonists are a type of medication that block the action of the CysLT1 receptor. This receptor is involved in the inflammatory response and is particularly important in conditions such as asthma and allergic rhinitis. By blocking the CysLT1 receptor, these antagonists help to reduce inflammation and alleviate symptoms associated with these conditions.
PDE3 inhibitors are a class of drugs that inhibit the enzyme phosphodiesterase type 3 (PDE3). This enzyme is involved in the breakdown of cyclic adenosine monophosphate (cAMP), a signaling molecule that regulates various cellular processes. By inhibiting PDE3, these drugs increase the levels of cAMP, leading to relaxation of smooth muscles, increased contractility of the heart, and other effects. PDE3 inhibitors are used in the treatment of conditions such as heart failure and intermittent claudication.
PDE4 inhibitors, on the other hand, are medications that inhibit the enzyme phosphodiesterase type 4 (PDE4). Similar to PDE3 inhibitors, PDE4 inhibitors also increase the levels of cAMP by blocking its breakdown. These drugs have anti-inflammatory properties and are used in the treatment of conditions such as chronic obstructive pulmonary disease (COPD) and psoriasis.
PGI2 receptor antagonists are drugs that block the action of the prostacyclin (PGI2) receptor. Prostacyclin is a naturally occurring substance in the body that helps to prevent blood clotting and promotes vasodilation (widening of blood vessels). By antagonizing the PGI2 receptor, these drugs reduce the effects of prostacyclin, which can be beneficial in conditions such as pulmonary arterial hypertension and Raynaud's phenomenon.
In summary, CysLT1 antagonists target a specific receptor involved in inflammation, PDE3 inhibitors and PDE4 inhibitors affect the breakdown of cAMP and have various effects on smooth muscles and inflammation, and PGI2 receptor antagonists block the action of a receptor involved in blood clotting and vasodilation.
Drug Target R&D Trends for Ibudilast
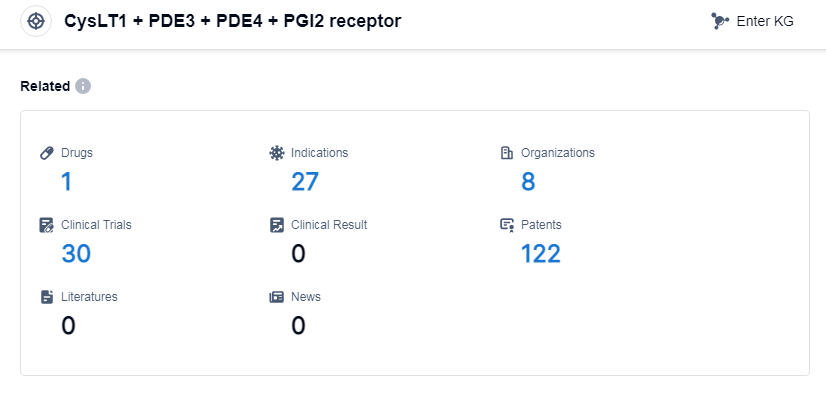
According to Patsnap Synapse, as of 11 Sep 2023, there are a total of 1 CysLT1, PDE3, PDE4 and PGI2 receptor drugs worldwide, from 8 organizations, covering 27 indications, and conducting 30 clinical trials.
Based on the analysis of the provided data, the current competitive landscape of the target CysLT1, PDE3, PDE4 and PGI2 receptor indicates active involvement of companies like MediciNova, Inc., KYORIN Pharmaceutical Co., Ltd., and Shenzhen Neptunus Pharmaceutical Co. Ltd. These companies are progressing in different stages of development, ranging from approved drugs to preclinical stages.
The target has shown success in treating indications like neuralgia, multiple sclerosis, cerebral hemorrhage, and asthma, among others. Ongoing research is exploring the potential of the target in various therapeutic areas, including spinal cord diseases, amyotrophic lateral sclerosis, and opioid-related disorders.
Small molecule drugs are the most rapidly progressing drug type under this target, indicating potential competition in the market.
Countries like Japan, South Korea, China, the United Kingdom, and the United States are actively involved in the development of drugs targeting the CysLT1, PDE3, PDE4 and PGI2 receptor.
Overall, the target CysLT1, PDE3, PDE4 and PGI2 receptor shows promise in terms of ongoing research and development, with potential for future advancements in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Ibudilast is a small molecule drug that targets multiple receptors and has a broad range of therapeutic areas. It has been approved for various indications, including neurological disorders, respiratory diseases, substance abuse disorders, and cancer. Developed by KYORIN Pharmaceutical Co., Ltd., it received its first approval in Japan in 1989 and is currently approved globally. Its orphan drug status highlights its potential for treating rare diseases.