Pharmaceutical Insights: Pralsetinib's R&D Progress and its Mechanism of Action
Pralsetinib's R&D Progress
Pralsetinib is a small molecule drug that falls under the therapeutic area of neoplasms, endocrinology and metabolic disease, and respiratory diseases. It specifically targets the RET protein, making it a potential treatment option for various cancers and related conditions.
The drug has shown efficacy in treating several indications, including non-small cell lung cancer (NSCLC), RET fusion-positive NSCLC, RET fusion-positive thyroid cancer, RET mutation-positive medullary thyroid cancer, advanced lung non-small cell carcinoma, thyroid cancer, and neoplasms. This broad range of indications highlights the potential versatility of Pralsetinib in addressing different types of cancers and related diseases.
Pralsetinib was developed by Blueprint Medicines Corp., an originator organization in the pharmaceutical industry. It has received approval for use in the highest phase globally. The drug obtained its first approval in the United States in September 2020, indicating its recent entry into the market.
Regulatory authorities have recognized the potential of Pralsetinib, as evidenced by its designation as a priority review, accelerated approval, orphan drug, and breakthrough therapy. These designations indicate that the drug has shown promising results in clinical trials and may provide significant benefits to patients with limited treatment options.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Pralsetinib: RET inhibitors
RET inhibitors are a type of medication that specifically target and inhibit the activity of the RET (rearranged during transfection) protein. The RET protein is a receptor tyrosine kinase that plays a crucial role in cell signaling pathways involved in cell growth, differentiation, and survival. When the RET protein is mutated or overexpressed, it can contribute to the development and progression of various cancers, particularly thyroid cancer and non-small cell lung cancer.
RET inhibitors work by binding to the RET protein and blocking its activity, thereby inhibiting the abnormal signaling pathways associated with cancer growth. By targeting RET, these inhibitors help to slow down or halt the proliferation of cancer cells and may also induce cell death. They are considered a promising therapeutic approach for patients with RET-driven cancers.
In the field of biomedicine, RET inhibitors are a significant advancement in precision medicine, as they specifically target the molecular abnormalities driving cancer growth. These inhibitors can be used as targeted therapies, either alone or in combination with other anticancer drugs, to improve treatment outcomes for patients with RET-driven cancers.
Drug Target R&D Trends for Pralsetinib
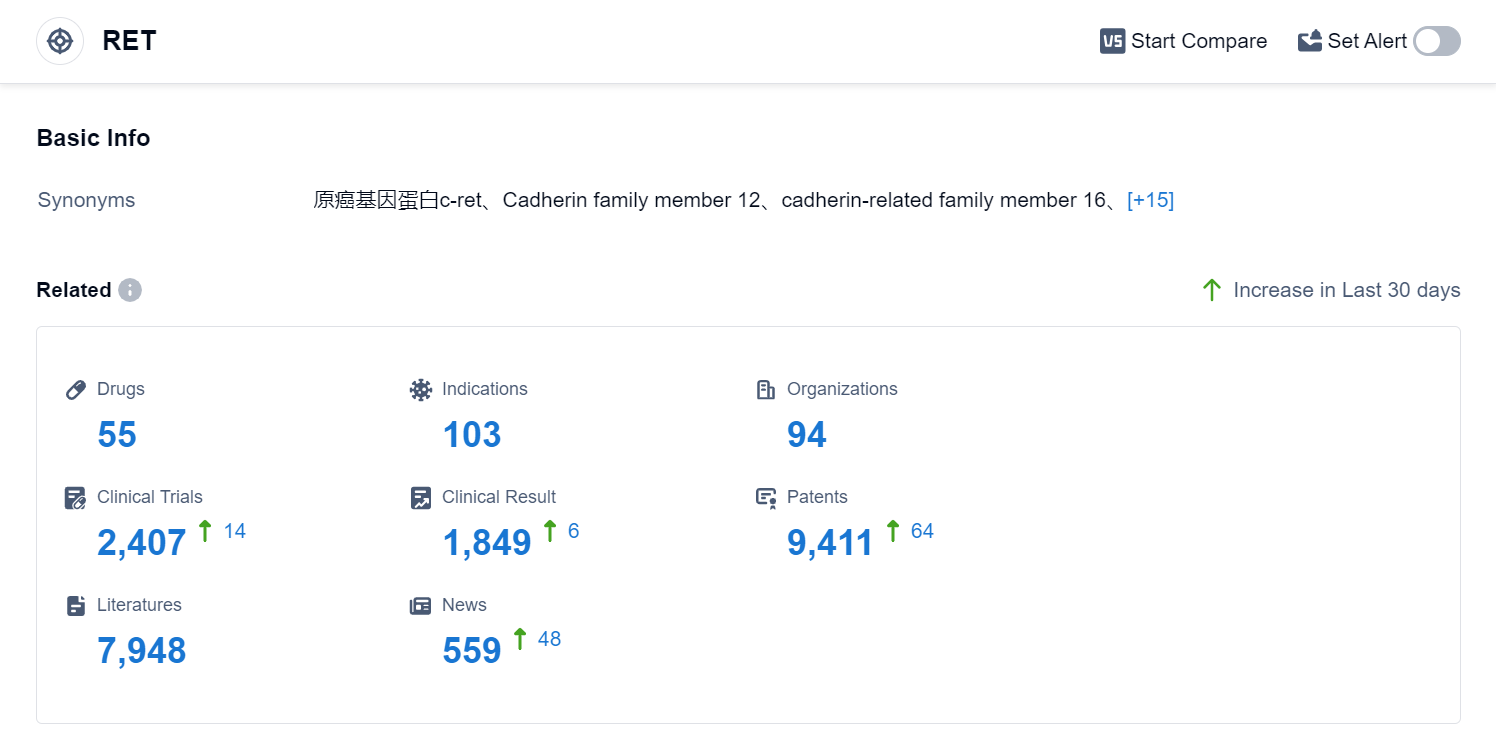
According to Patsnap Synapse, as of 2 Sep 2023, there are a total of 55 RET drugs worldwide, from 94 organizations, covering 103 indications, and conducting 2407 clinical trials.
The analysis of the current competitive landscape and future development of target RET reveals that multiple companies are actively involved in the research and development of drugs targeting RET. Bayer AG, Roche Holding AG, and Eisai Co., Ltd. are leading in terms of the highest stage of development. The indications for the drugs under target RET cover a wide range of cancers, indicating the potential for significant impact in the field of oncology. Small molecule drugs are progressing rapidly, suggesting intense competition and the need for innovative solutions. China has shown notable progress in the development of drugs under target RET, indicating its growing presence in the pharmaceutical industry. Overall, the target RET presents a promising opportunity for companies and researchers in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Pralsetinib is a small molecule drug developed by Blueprint Medicines Corp. It targets the RET protein and has been approved for use in various indications related to neoplasms, endocrinology and metabolic disease, and respiratory diseases. Its first approval was obtained in the United States in September 2020, and it has received regulatory designations that highlight its potential as a breakthrough therapy. Pralsetinib represents a promising treatment option for patients with specific types of cancers and related conditions.