Zenas BioPharma publicizes significant licensing and teamwork contract with Bristol Myers Squibb, aiming to market the innovative bi-functional antibody Obexelimab
Zenas BioPharma, an international biopharmaceutical entity dedicated to pivoting into a preeminent role in evolving and selling immunology-centric therapies, disclosed today their signing of a licensing and partnership contract with Bristol Myers Squibb Company. The intent is to evolve and sell obexelimab for autoimmune ailments in regions including Japan, South Korea, Taiwan, Singapore, Hong Kong, and Australia.
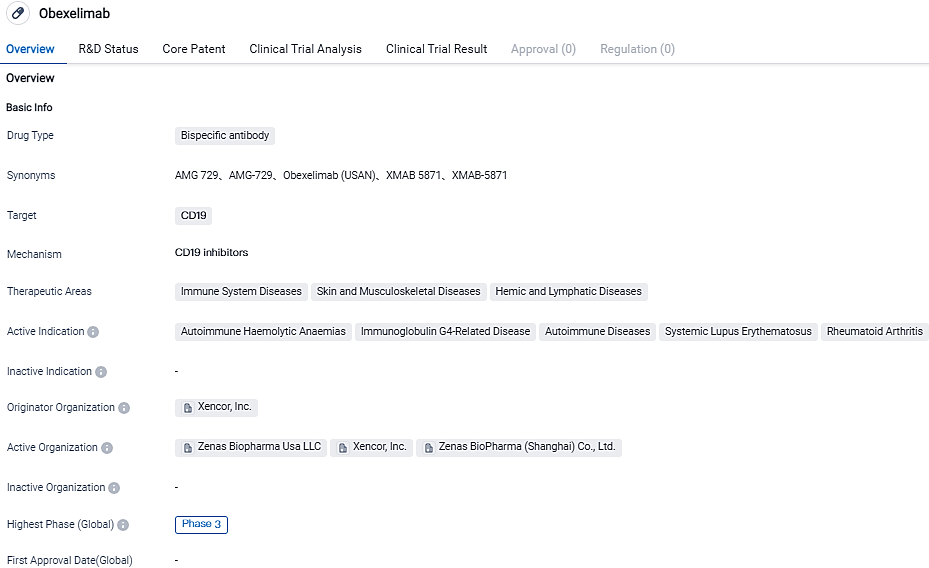
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Obexelimab is a bifunctional, non-cytolytic, humanized monoclonal antibody. It works by binding to CD19 and FcγRIIb to suppress B cells, plasmablasts, and plasma cells expressing CD19. A global Phase III trial is currently studying obexelimab's potential as a subcutaneous injection for patients diagnosed with IgG4-Related Disease. Additionally, obexelimab is being examined in a worldwide Phase II/III trial for patients suffering from warm antibody Auto-Immune Hemolytic Anemia.
Lonnie Moulder, founder and CEO of Zenas BioPharma stated that this cooperation plays a key role in furthering their objective of offering obexelimab benefits to patients dealing with auto immune diseases globally. He added that the collaboration with Bristol Myers Squibb's experienced and dedicated team was excellent, given their success in developmental, regulatory and commercial aspects.
Steve Sugino, Senior Vice President & General Manager at Japan, Bristol Myers Squibb expressed excitement to work alongside Zenas BioPharma to enhance obexelimab's influence in the licensed territory. He indicated that this partnership offers a significant chance to cater to those suffering from IgG4-RD, as there are no approved treatment options available currently.
As the license agreement's terms dictate, Zenas will obtain a $50 million initial cash payment and is also eligible to receive supplementary payments upon accomplishing certain development, regulatory, and commercial milestones. Additionally, royalties will be collected on obexelimab's net sales. In return, Bristol Myers Squibb gains exclusive rights for obexelimab's development and commercialization in the designated zone.
Obexelimab, a humanized monoclonal antibody currently in its investigational Phase 3 stage, imitates antigen-antibody complex behaviors to inhibit B-lineage cells by binding to CD19 and FcγRIIb. Various early-stage clinical studies wherein 198 subjects were treated with obexelimab, demonstrated promising results in patients with a range of autoimmune diseases. These studies showed that obexelimab could suppress B cell functionality without cell depletion.
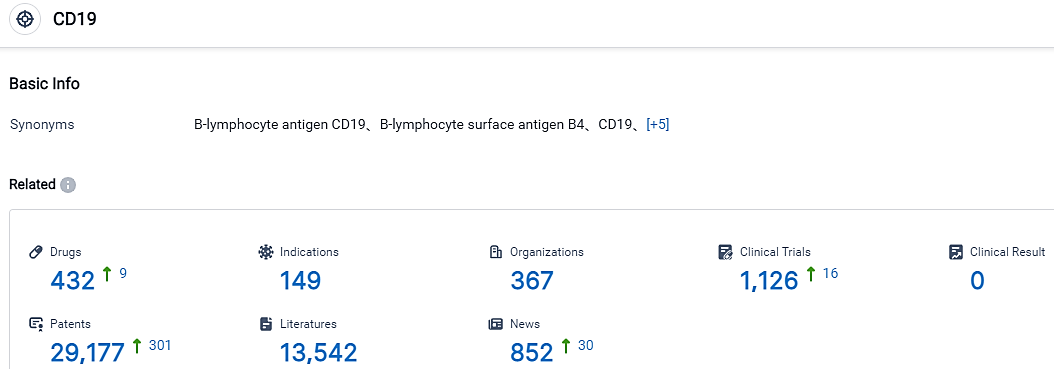
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 6, 2023, there are 432 investigational drugs for the CD19 target, including 149 applicable indications, 367 R&D institutions involved, with related clinical trials reaching 1126, and as many as 29177 patents.
Globally, glucocorticoids are commonly regarded as the definitive treatment for IgG4-RD, for which there are currently no approved treatments. Despite their frequent use, glucocorticoids and existing B cell depleting therapies rarely result in lasting, treatment-free remissions and often correlate with heightened risk of toxicity in patients. These treatments might also impede responses to vaccinations, such as those for SARS-CoV-2 and the flu.