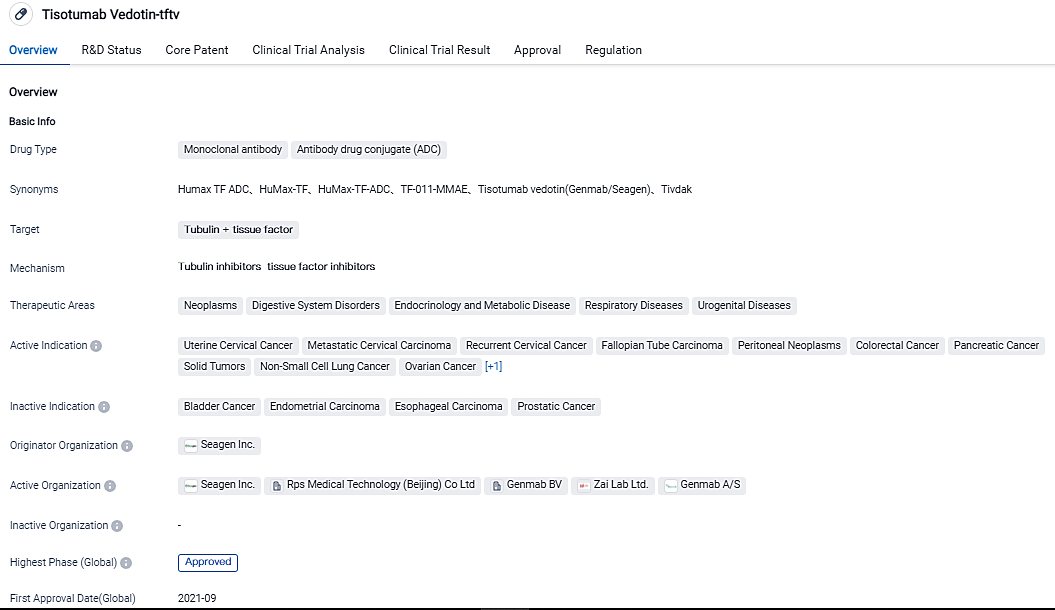
Genmab and Seagen report that TIVDAK® achieved its main objective of enhancing overall survival rates in patients suffering from recurrent or metastatic cervical cancer
Genmab A/S and Seagen Inc. revealed that the phase 3 innovaTV 301 worldwide study involving patients with recurrent or metastatic cervical cancer, whose disease advanced after receiving initial treatment, met its main objective of overall survival when TIVDAK®(tisotumab vedotin-tftv) was used in comparison to chemotherapy only.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
An Independent Data Monitoring Committee affirmed that OS surpassed the predetermined efficiency limit during the interim evaluation. Significant statistics were also displayed from the secondary key endpoints of investigator-evaluated progression-free survival and objective response rate. TIVDAK’s safety profile within innovaTV 301 mirrors its known safety profile as outlined in the U.S. prescription data, with no new safety concerns identified.
The latter provided the foundation for the fast-tracked approval of TIVDAK in the United States. Subject to conversations with regulatory bodies, the findings of innovaTV 301 are slated to serve as the defining confirmatory trial for TIVDAK's rapid U.S. approval and endorsement of global regulatory applications. Collaborating with Zai Lab Limited, the extension study of innovaTV 301 in China has begun and is actively recruiting patients.
"TIVDAK stands as the single therapy approved by the FDA for second-line recurrent or metastatic cervical cancer, irrespective of biomarker status, tumor histology, and previous treatment," stated Roger Dansey, M.D., President of Research and Development and Chief Medical Officer at Seagen.
"For patients with advanced cervical cancer who have experienced progression after initial treatment, therapeutic options are limited, emphasizing the demand for treatments with innovative mechanisms particularly those offering a survival advantage," noted Jan van de Winkel, Ph.D., Chief Executive Officer, Genmab. "These results deliver a glimmer of hope for patients living with recurrent or metastatic cervical cancer."
TIVDAK®(tisotumab vedotin) is an antibody-drug conjugate composed of a human monoclonal antibody directed at tissue factor from Genmab and a protease-breakable linker that covalently binds Seagen’s microtubule-destabilizing agent monomethyl auristatin E to the antibody.
Non-laboratory data suggest that TIVDAK's anticancer activity is due to the ADC binding with cancer cells expressing TF, followed by the ADC-TF complex's intake and subsequent discharge of MMAE via proteolytic splitting. MMAE disrupts the microtubule network in cells undergoing active division, causing cell cycle cessation and apoptosis.
In September 2021, TIVDAK received fast-track approval from the U.S. Food and Drug Administration for adults with recurring or metastatic cervical cancer that has progressed during or post-chemotherapy. TIVDAK is the first and only approved ADC for these patients.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
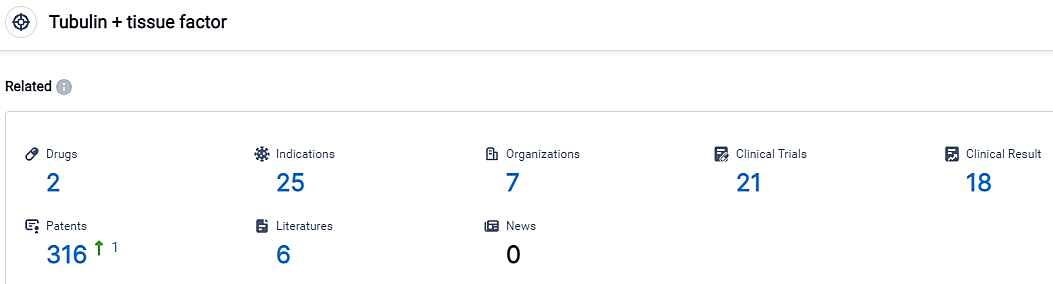 According to the data provided by the Synapse Database, As of September 6, 2023, there are 2 investigational drugs for the Tubulin and tissue factor target, including 25 applicable indications, 7 R&D institutions involved, with related clinical trials reaching 21, and as many as 316 patents.
According to the data provided by the Synapse Database, As of September 6, 2023, there are 2 investigational drugs for the Tubulin and tissue factor target, including 25 applicable indications, 7 R&D institutions involved, with related clinical trials reaching 21, and as many as 316 patents.
Despite improvements in the effectiveness of vaccinations and screening processes to help prevent and detect pre-/early-stage cervical cancer for the possibility of a cure, the disease still poses a significant challenge that is yet to be adequately addressed. Predictions for the year 2023 suggest that over 13,960 fresh instances of this aggressive form of cervical cancer will be identified in the U.S., and the disease will be responsible for the death of at least 4,310 adults.