AHR Agonists -The Magic Multitasker of Immune Regulation
The Aryl Hydrocarbon Receptor (AHR) is a transcriptional regulatory factor in cells that can bind to ligands. Normally, AHR exists in the cytoplasm, forming a complex with various companion proteins. However, upon binding with a ligand, AHR enters into the cell nucleus through the nuclear pore and forms a dimer with the Aryl Hydrocarbon Receptor Nuclear Translocator (ARNT). This formed dimer can bind with the Xenobiotic response element (XRE) in DNA, promoting the expression of downstream genes. In the nucleus, AHR can also bind with other transcription factors, thereby affecting the expression of genes regulated by the bound transcription factors.
Among the proteins regulated by the AHR signaling pathway, the cytochrome P450 superfamily is the most thoroughly researched. The activation of AHR can directly trigger the expression of proteins like CYP1A1, CYP1A2, and these enzymes play a crucial role in the metabolism of aromatic compounds: they are responsible not only for the metabolism of exogenous toxic substances but also impact the biotransformation of various drugs.
AHR acts as a sensor for cells to perceive the external environment, with high expression in barrier cells and certain immune cells. The AHR signaling pathway has also been proven to be a key signaling pathway in the regulation of the immune system. AHR agonists have shown effects in some representative models of autoimmune diseases (including systemic lupus erythematosus, Behcet's syndrome, multiple sclerosis, inflammatory bowel disease, etc.).
AHR Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 4 Sep 2023, there are a total of 19 AHR drugs worldwide, from 28 organizations, covering 22 indications, and conducting 49 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
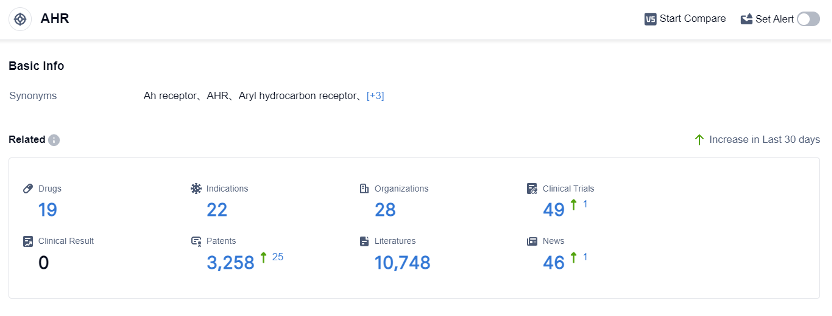
The analysis of target AHR reveals a competitive landscape with multiple companies actively involved in the development of drugs targeting AHR. Roivant Sciences Ltd., Guangdong Zhiguang Biotechnology Co. Ltd., and Shenzhen Tianji Pharmaceutical Co Ltd. are among the fastest-growing companies in this field.
The indications for AHR-related drugs cover a wide range of diseases, including psoriasis, dermatitis, inflammatory bowel diseases, neoplasms, and more. Small molecule drugs and chemical drugs are the most rapidly progressing drug types, indicating intense competition in the development of innovative drugs.
China has shown progress in the development of AHR-related drugs, alongside the United States, which remains the leading country in this field. Overall, the target AHR presents a promising area for pharmaceutical development with diverse indications and active research and development efforts worldwide.
Approved AHR agonists for market launch:Benvitimod
Benvitimod is a small molecule drug developed by Guangdong Zhonghao Pharmaceutical Co. Ltd. It is primarily used for the treatment of immune system diseases, congenital disorders, and skin and musculoskeletal diseases. The drug targets the AHR, which plays a crucial role in regulating immune responses and inflammation.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
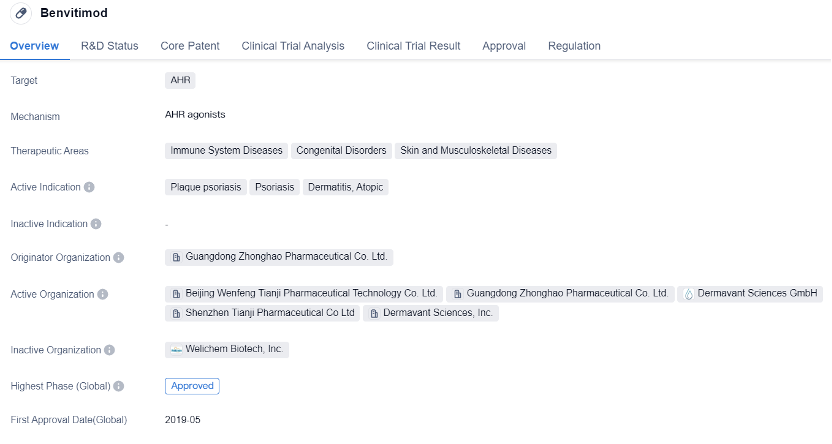
The active indications for Benvitimod include plaque psoriasis, psoriasis, dermatitis, and atopic dermatitis. These conditions are characterized by chronic inflammation and abnormal immune responses, leading to symptoms such as redness, itching, and scaling of the skin. By targeting the AHR, Benvitimod aims to modulate the immune system and reduce inflammation, providing relief to patients suffering from these conditions.
Benvitimod has received approval for use in both global and Chinese markets. The drug obtained its first approval in China in May 2019, making it available to patients in the country. The approval process for Benvitimod involved special review projects and priority review, indicating the potential significance and urgency of the drug in addressing the unmet medical needs of patients with immune system diseases and skin disorders.
As a small molecule drug, Benvitimod offers several advantages in terms of formulation, administration, and potential for oral delivery. Small molecule drugs are typically easier to manufacture and have a well-established regulatory pathway for approval. This may contribute to the drug's successful development and approval in both global and Chinese markets.
AHR agonists entering into phase I clinical trials:AT-177
AT-177 is a small molecule drug that is being developed by Azora Therapeutics, Inc. It is designed to target the AHR and is intended for the treatment of digestive system disorders. The drug is currently in Phase 1 of clinical development, which is the earliest stage of testing in humans.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
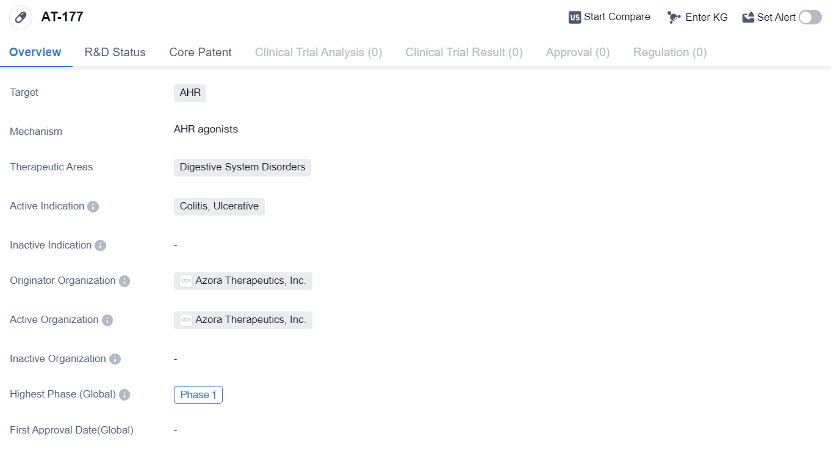
The therapeutic areas that AT-177 is focused on are digestive system disorders, specifically colitis and ulcerative colitis. Colitis refers to inflammation of the colon, while ulcerative colitis is a chronic inflammatory bowel disease that affects the colon and rectum. These conditions can cause symptoms such as abdominal pain, diarrhea, and rectal bleeding.
Being in Phase 1 of clinical development means that AT-177 is still in the early stages of testing in humans. Phase 1 trials are typically conducted with a small number of healthy volunteers or patients to evaluate the safety, dosage, and potential side effects of the drug. These trials are important for determining the appropriate dosage and identifying any potential risks or adverse reactions.
In summary, AT-177 is a small molecule drug being developed by Azora Therapeutics, Inc. for the treatment of digestive system disorders, specifically colitis and ulcerative colitis. It is currently in Phase 1 of clinical development, and its target is the AHR. Further research and clinical trials will be necessary to assess the drug's potential effectiveness and safety.