Pharmaceutical Insights: Vardenafil hydrochloride's R&D Progress
Vardenafil hydrochloride's R&D Progress
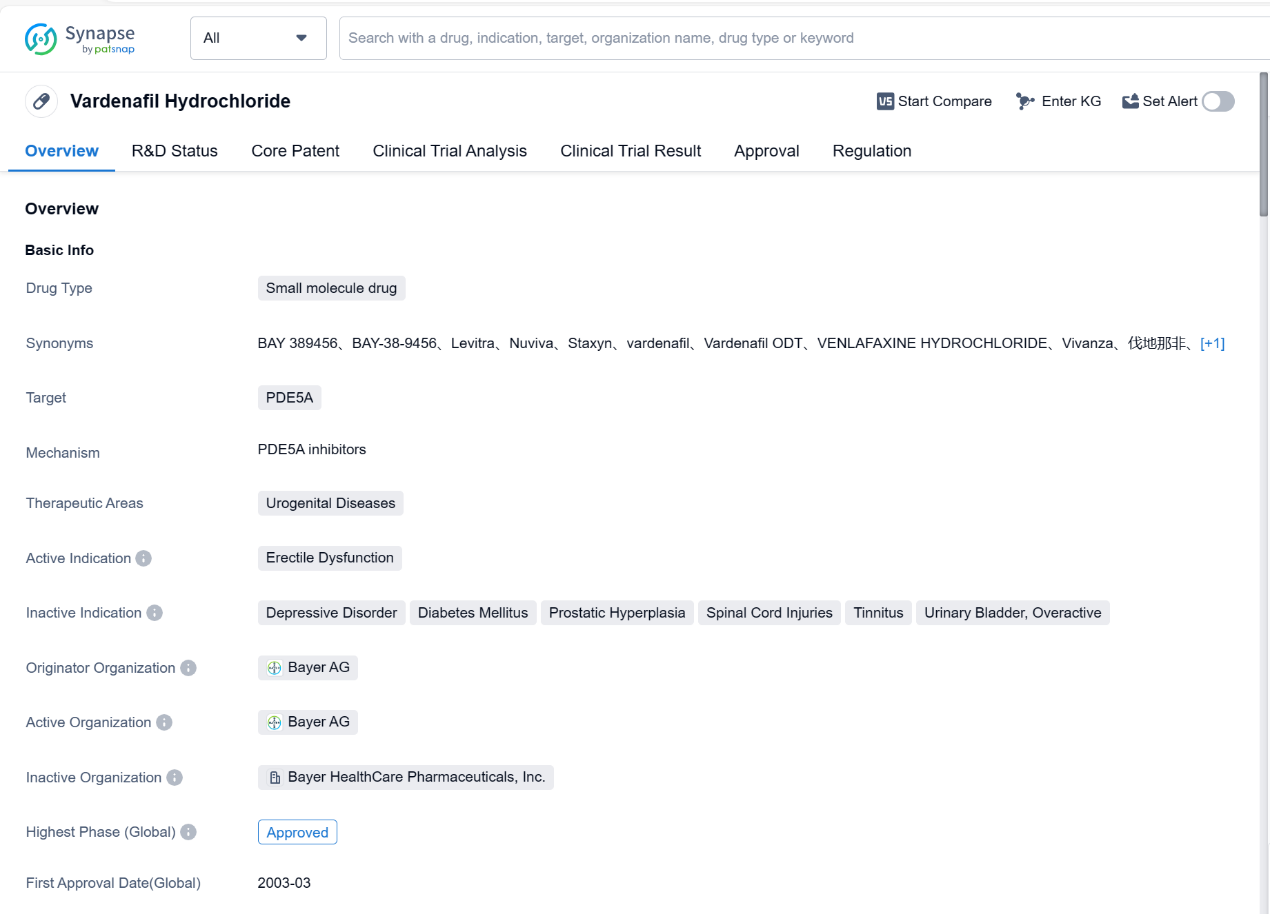
Vardenafil Hydrochloride is a small molecule drug that falls under the therapeutic area of urogenital diseases. It specifically targets the enzyme PDE5A. The drug is primarily indicated for the treatment of erectile dysfunction.
The originator organization of Vardenafil Hydrochloride is Bayer AG, a well-known pharmaceutical company. The drug has reached the highest phase of approval both globally and in China. It received its first approval in the European Union in March 2003.
Vardenafil Hydrochloride is classified as a small molecule drug, which means it consists of low molecular weight compounds. This classification is important as it determines the drug's properties, such as its ability to penetrate cell membranes and interact with specific targets.
The drug's target, PDE5A, is an enzyme that plays a crucial role in regulating blood flow. By inhibiting this enzyme, Vardenafil Hydrochloride helps to relax the smooth muscles in the penis, allowing for increased blood flow and thus facilitating an erection. This mechanism of action has made it an effective treatment for erectile dysfunction.
The approval of Vardenafil Hydrochloride in both the global and Chinese markets indicates its safety and efficacy profile has met the necessary regulatory requirements. The drug has undergone a priority review, which suggests that it may have demonstrated significant therapeutic benefits or addressed an unmet medical need.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Vardenafil hydrochloride: PDE5A inhibitors
PDE5A inhibitors are a type of medication that blocks the action of the enzyme phosphodiesterase type 5A (PDE5A). This enzyme is primarily found in the smooth muscle cells of blood vessels, including those in the penis, lungs, and other organs. By inhibiting PDE5A, these medications promote the relaxation of smooth muscle cells and increase blood flow to specific areas of the body.
From a biomedical perspective, PDE5A inhibitors are commonly used to treat erectile dysfunction (ED) in men. They work by enhancing the effects of nitric oxide, a chemical that is released during sexual stimulation and causes the relaxation of smooth muscles in the penis. This increased blood flow helps to facilitate and maintain an erection.
PDE5A inhibitors are also used in the treatment of pulmonary arterial hypertension (PAH), a condition characterized by high blood pressure in the arteries of the lungs. By dilating the blood vessels in the lungs, these medications help reduce the workload on the heart and improve exercise capacity in individuals with PAH.
Some examples of PDE5A inhibitors include sildenafil (Viagra), tadalafil (Cialis), and vardenafil (Levitra). It is important to note that these medications should be used under the guidance of a healthcare professional, as they may have potential side effects and interactions with other medications.
Drug Target R&D Trends for Vardenafil hydrochloride
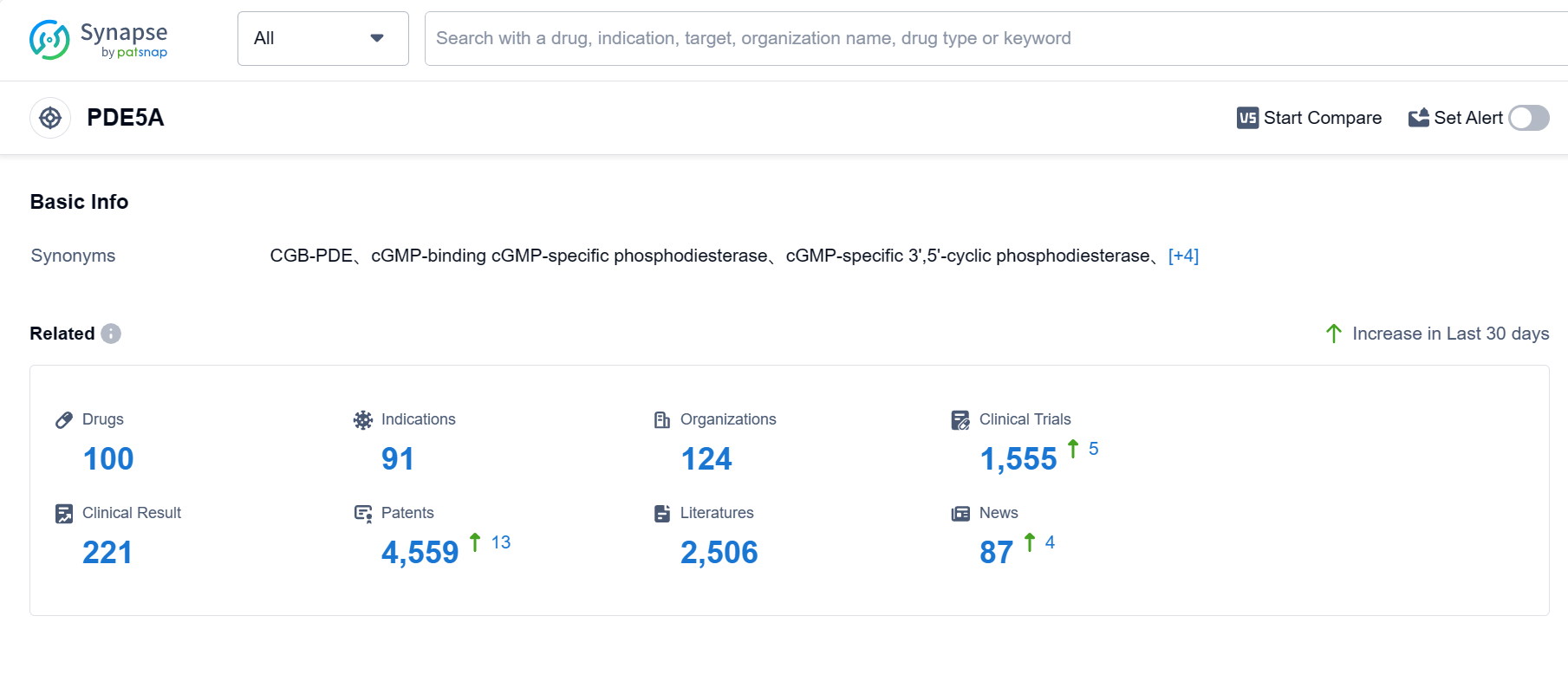
PDE5A, also known as phosphodiesterase type 5A, is an enzyme found in the human body that plays a crucial role in regulating the levels of cyclic guanosine monophosphate (cGMP). cGMP is responsible for relaxing the smooth muscles in blood vessels, including those in the penis. PDE5A breaks down cGMP, leading to vasoconstriction and decreased blood flow. Inhibiting PDE5A with medications like Viagra or Cialis can increase cGMP levels, promoting vasodilation and improving blood flow, particularly in the penile region. This mechanism is utilized to treat erectile dysfunction and pulmonary arterial hypertension, where PDE5A inhibition helps restore normal blood flow and improve patient outcomes. According to Patsnap Synapse, as of 4 Sep 2023, there are a total of 100 PDE5A drugs worldwide, from 124 organizations, covering 91 indications, and conducting 1555 clinical trials.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Vardenafil Hydrochloride is a small molecule drug developed by Bayer AG for the treatment of erectile dysfunction. Its approval in multiple markets and its specific targeting of PDE5A make it a valuable option for patients suffering from this condition.