Promising Early Results from Johnson & Johnson's Phase 3 & 2 Trials for Generalized Myasthenia Gravis and Sjögren's Disease Treatments
Johnson & Johnson has disclosed primary outcomes from the crucial Phase 3 clinical trial, termed VIVACITY, researching the effects of nipocalimab on adults diagnosed with generalized myasthenia gravis. Additionally, initial findings from the Phase 2 investigation known as DAHLIAS, which is evaluating the efficacy of nipocalimab in treating adults affected by Sjögren's disease, were also shared.
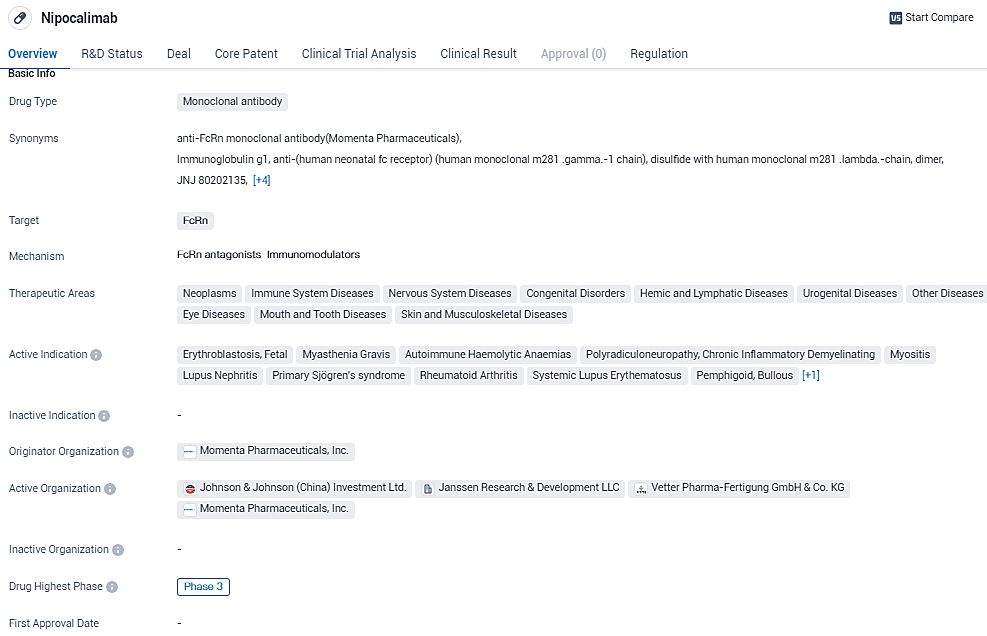
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
In the prior 12 months, the therapeutic potential of Nipocalimab has been affirmed in the management of four distinct diseases where autoantibodies play a pivotal role. These conditions include the fetal and neonatal hemolytic disorder and rheumatoid arthritis, as well as gMG and SjD.
During the pivotal Phase 3 VIVACITY trial for gMG, Nipocalimab achieved its primary objective by reporting a marked and statistically significant decrease in the MG-ADL score at the 22 to 24-week mark when assessed against a placebo. gMG is a chronic, rare neuromuscular disorder marked by varying degrees of muscle weakness, which persists throughout the patient's life and leads to significant disability.
Similar success was seen in the Phase 2 DAHLIAS trial, tailored for SjD, where patients exhibited a significant drop in the clinESSDAI score at the 24-week threshold relative to placebo administration. This constitutes the first instance of a positive outcome for an FcRn antagonist under investigation in SjD, a chronic and disabling autoantibody-mediated condition that currently lacks approved cutting-edge treatments.
The prevalence of SjD is notably higher in the female population, being approximately nine times more frequent when compared to males. This demographic detail underscores the importance of Nipocalimab, as this investigational anti-FcRn has shown a favorable benefit-risk profile, particularly in pregnant subjects involved in clinical assessments.
Looking forward, Johnson & Johnson is preparing to disclose the entire findings from the VIVACITY Phase 3 study at a forthcoming medical scientific conference and will commence discussions with worldwide health regulators regarding the introduction of Nipocalimab for patients afflicted with gMG. The insights from the DAHLIAS Phase 2 study bolster the case for Nipocalimab's continued clinical advancement in the context of SjD, and a comprehensive disclosure of these study findings is anticipated to occur at a medical congress this year.
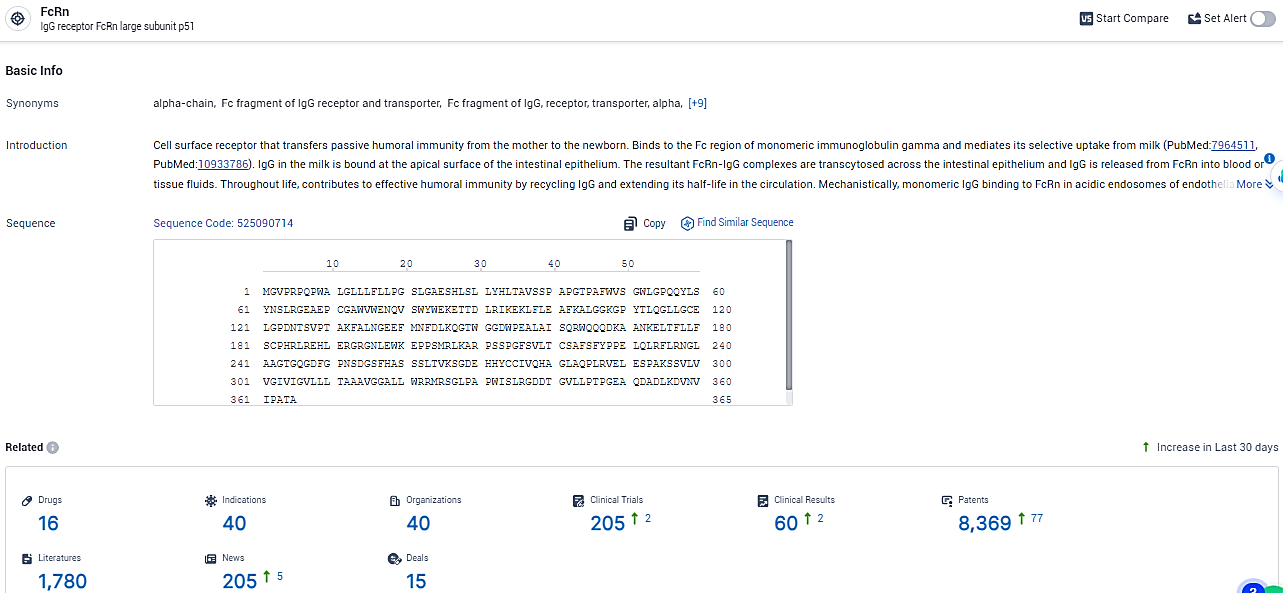
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of February 18, 2024, there are 16 investigational drugs for the FcRn target, including 40 indications, 40 R&D institutions involved, with related clinical trials reaching 205, and as many as 8369 patents.
Nipocalimab shows promise as a monoclonal antibody targeting FcRn for the treatment of various diseases. Its broad therapeutic areas and active indications suggest its potential to address multiple medical conditions. The drug's advanced stage of development and regulatory designations further support its potential for future success in the pharmaceutical industry.