Potential treatment drugs for autoimmune diseases - IL-17 inhibitors
IL-17, or Interleukin-17, is a cytokine that plays a crucial role in the human body's immune response. It is primarily produced by a subset of T cells called Th17 cells. IL-17 acts as a pro-inflammatory mediator, promoting the recruitment and activation of immune cells to sites of infection or tissue damage. It is involved in various immune-related processes, including the defense against pathogens, regulation of autoimmune responses, and tissue inflammation. Dysregulation of IL-17 has been implicated in several autoimmune diseases, such as psoriasis and rheumatoid arthritis. Understanding the role of IL-17 is essential for developing targeted therapies to modulate immune responses and treat related disorders.
IL-17 Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 20 Sep 2023, there are a total of 26 IL-17 drugs worldwide, from 27 organizations, covering 27 indications, and conducting 27 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.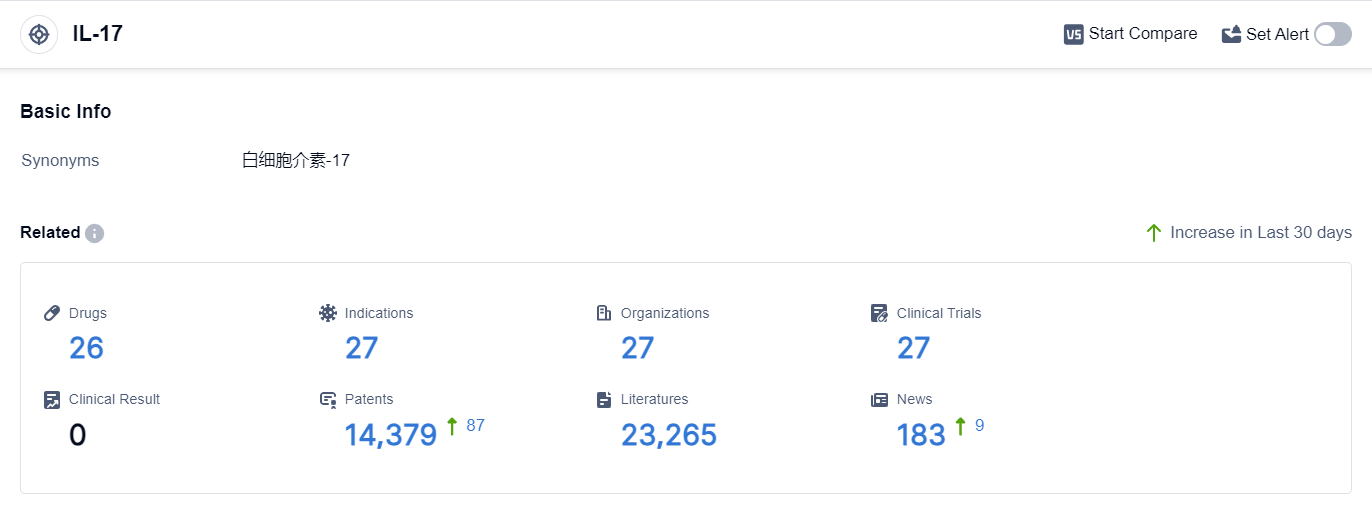
The analysis of target IL-17 reveals a competitive landscape with multiple companies actively involved in the research and development of IL-17 inhibitors. Akeso, Inc., Eli Lilly & Co., and Zhejiang Huahai Pharmaceutical Co., Ltd. are among the companies growing fastest under the current target.
The indications for IL-17 inhibitors cover a wide range of diseases, indicating their potential in various therapeutic areas. Monoclonal antibodies and small molecule drugs are the drug types progressing most rapidly under the current target.
China, the United States, and the European Union are the countries/locations developing fastest under the target IL-17, with China showing significant progress. Overall, the target IL-17 presents a promising opportunity for the development of innovative therapies in the pharmaceutical industry.
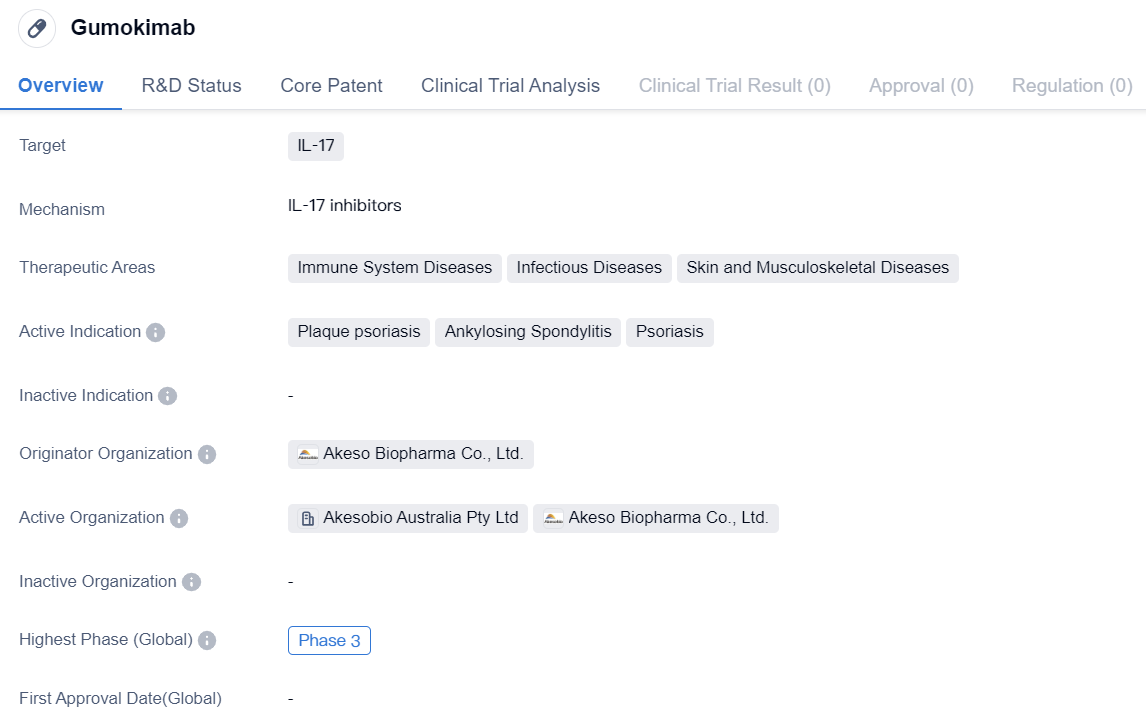
IL-17 inhibitors entering Phase III clinical trials: Gumokimab
Gumokimab is a monoclonal antibody drug that targets IL-17, a protein involved in immune system regulation. It is being developed by Akeso Biopharma Co., Ltd., a pharmaceutical company specializing in biomedicine. The drug is currently in Phase 3 of clinical trials, both globally and in China.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The therapeutic areas of Gumokimab include immune system diseases, infectious diseases, and skin and musculoskeletal diseases. It is specifically indicated for the treatment of plaque psoriasis, ankylosing spondylitis, and psoriasis. These conditions are characterized by chronic inflammation and can significantly impact patients' quality of life.
As a monoclonal antibody, Gumokimab is designed to bind to IL-17 and inhibit its activity. IL-17 is known to play a role in the inflammatory response, and by targeting this protein, Gumokimab aims to reduce inflammation and alleviate symptoms associated with the aforementioned diseases.
The fact that Gumokimab has reached Phase 3 of clinical trials indicates that it has shown promising results in earlier stages of development. Phase 3 trials are large-scale studies involving a larger number of participants to further evaluate the drug's safety and efficacy. This phase is crucial in determining whether the drug will be approved for commercialization.
In summary, Gumokimab is a monoclonal antibody drug targeting IL-17, being developed by Akeso Biopharma Co., Ltd. It is currently in Phase 3 of clinical trials and shows potential for treating immune system diseases, infectious diseases, and skin and musculoskeletal diseases such as plaque psoriasis, ankylosing spondylitis, and psoriasis. Further research and evaluation will determine the drug's safety and efficacy, ultimately determining its potential for commercialization.
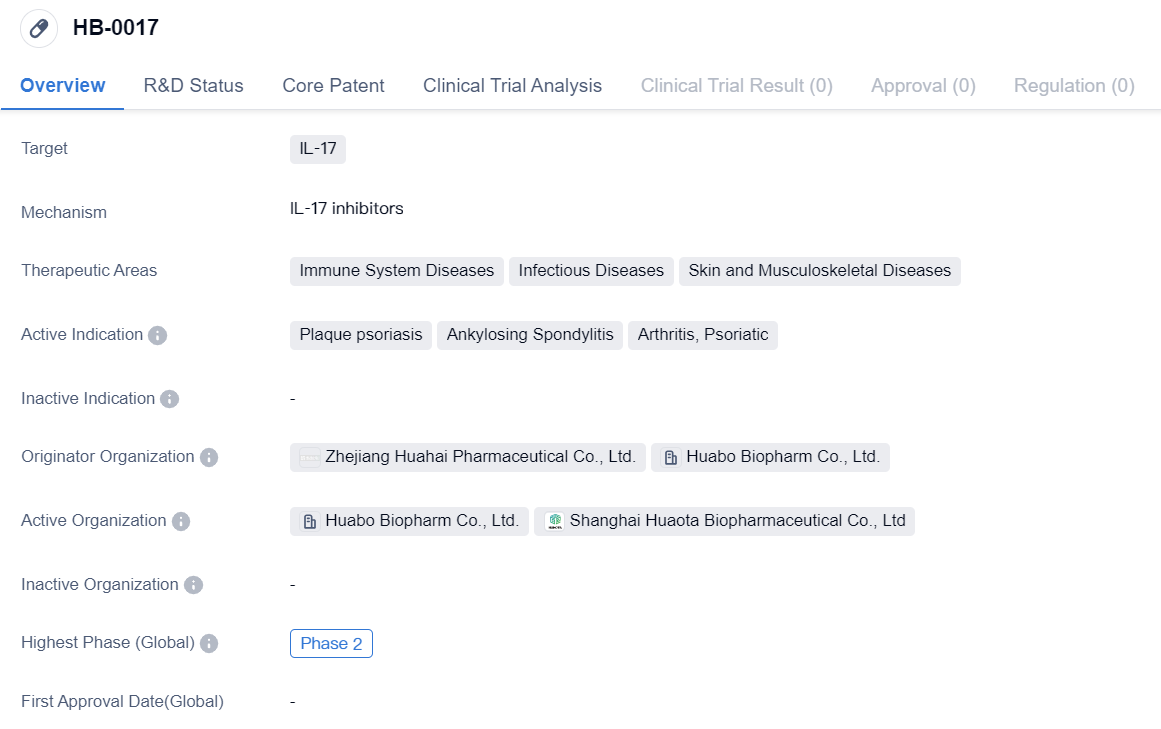
IL-17 inhibitors entering Phase II clinical trials: HB-0017
HB-0017 is a monoclonal antibody drug that targets IL-17, a protein involved in immune system regulation. The drug is being developed for the treatment of various diseases related to the immune system, infectious diseases, and skin and musculoskeletal diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The active indications for HB-0017 include plaque psoriasis, ankylosing spondylitis, arthritis, and psoriatic arthritis. These conditions are characterized by inflammation and immune system dysregulation, leading to symptoms such as skin lesions, joint pain, and stiffness.
The drug is being developed by Zhejiang Huahai Pharmaceutical Co., Ltd. and Huabo Biopharm Co., Ltd., both of which are originator organizations involved in the development and production of pharmaceutical products.
In terms of clinical development, HB-0017 has reached Phase 2 globally and in China. Phase 2 trials involve testing the drug's safety and efficacy in a larger group of patients to further evaluate its potential benefits and side effects. This suggests that HB-0017 has shown promising results in earlier stages of development and is now being tested in a larger patient population.
The development of monoclonal antibody drugs targeting IL-17 is an area of active research in the pharmaceutical industry. IL-17 plays a crucial role in the immune response and has been implicated in various immune-mediated diseases. By targeting IL-17, HB-0017 aims to modulate the immune system and alleviate symptoms associated with immune system dysregulation.
Overall, HB-0017 is a monoclonal antibody drug targeting IL-17 that is being developed for the treatment of immune system diseases, infectious diseases, and skin and musculoskeletal diseases. With its active indications including plaque psoriasis, ankylosing spondylitis, arthritis, and psoriatic arthritis, HB-0017 shows potential in addressing unmet medical needs in these therapeutic areas. The drug has reached Phase 2 of clinical development globally and in China, indicating promising results in earlier stages of testing. The development of IL-17-targeting monoclonal antibodies represents an important area of research in the pharmaceutical industry, aiming to provide new treatment options for patients suffering from immune-mediated diseases.