GALDERMA 2024: Phase III RelabotulinumtoxinA Trials Show Promising Results for Treating Facial Lines
Galderma recently shared encouraging preliminary outcomes from the phase III READY-3 clinical trial, which examined the efficacy of RelabotulinumtoxinA in managing frown lines and crow's feet — the clinical terms for glabellar and lateral canthal lines, respectively. The trial looked at the effects when the product was administered independently or in combination for treating these areas.
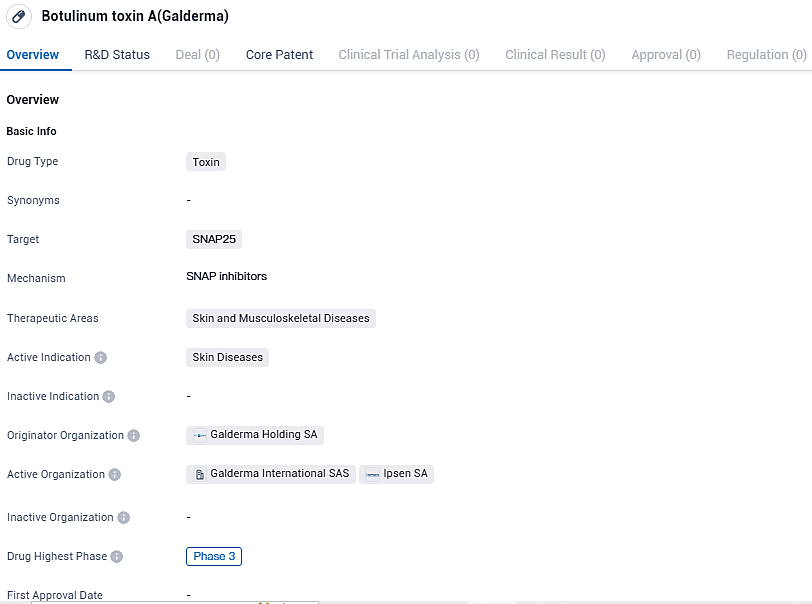
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Research outcomes confirm that a single administration of RelabotulinumtoxinA delivers a notable enhancement to both glabellar lines and lateral canthal lines, whether addressed separately or concurrently, maintaining effects up to a half-year period. These findings, along with additional discoveries from Galderma's extensive and pioneering aesthetic product range, are slated for display at the upcoming TOXINS 2024 International Convention in Berlin, from January 17 through January 20, 2024.
Excitement abounds regarding the impending dissemination of the latest breakthroughs from our prominent injectable aesthetic range at TOXINS 2024, specifically sharing initial insights from the phase III READY-3 study. These developments augment previous observations from the READY-1 and READY-2 studies, corroborating the sustained benefits of RelabotulinumtoxinA for treating glabellar and lateral canthal lines together.
The READY series of clinical investigations comprises four separate phase III studies that integrated in excess of 1,900 subjects. The READY-3 trial, a phase III research, was arranged in a randomized, double-blinded, and placebo-managed manner to evaluate the safety profile and therapeutic success of RelabotulinumtoxinA, as determined by aesthetic augmentation in individuals with moderate to intense glabellar lines or lateral canthal lines. The trial considered effects of the neurotoxin when used on each area individually or both concurrently, relative to a placebo control.
Dr. Baldassare Scassellati Sforzolini, serving as the Global Head of R&D at Galderma, expressed, "Patients frequently express a preference for aesthetics that sustain over time or wish for simultaneous treatments across several facial zones, while clinicians aim to provide reliable outcomes for their clientele. The recent findings underscore the efficacy of RelabotulinumtoxinA in satisfying these fundamental demands for treating glabellar and lateral canthal lines. Additionally, it offers a ready-to-use solution that practitioners find effortless to apply.”
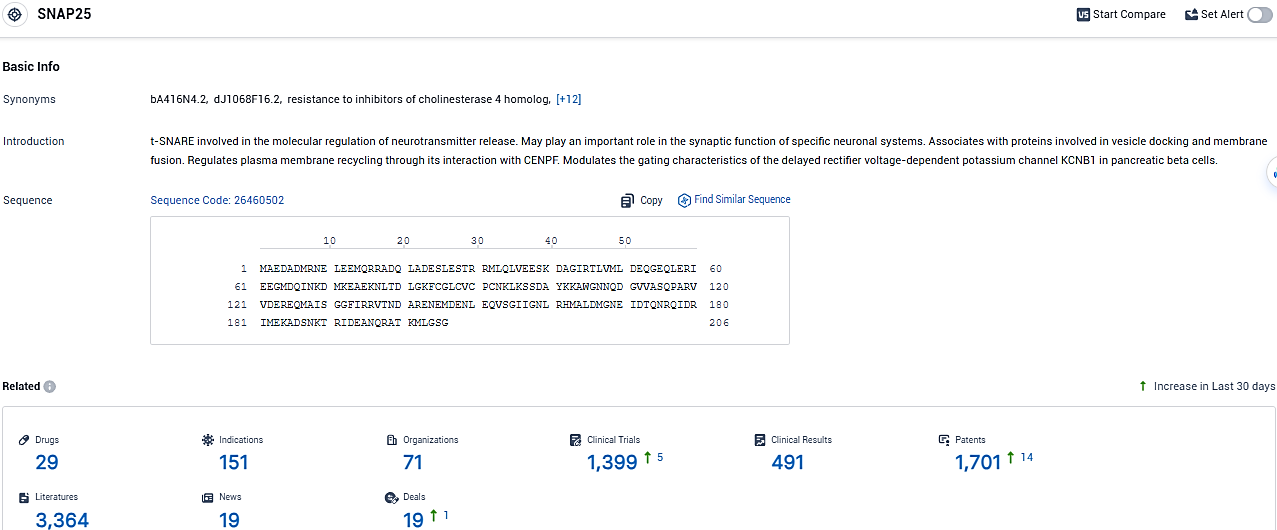
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of January 23, 2024, there are 29 investigational drugs for the SNAP25 target, including 151 indications, 71 R&D institutions involved, with related clinical trials reaching 491, and as many as 1701 patents.
RelabotulinumtoxinA, developed by Galderma Holding SA, is a toxin-based drug that targets SNAP25. RelabotulinumtoxinA is a highly-active, innovative, complex-free, and ready-to-use liquid botulinum toxin A with a proprietary strain and manufactured using a unique state-of-the-art process. RelabotulinumtoxinA is currently being investigated globally by Galderma, to expand its neuromodulator portfolio as part of the broadest Injectable Aesthetics portfolio on the market.