Progress in the Research of MEK Inhibitors
MEK, or Mitogen-Activated Protein Kinase Kinase, plays a crucial role in the human body as a key component of the MAPK signaling pathway. This pathway regulates various cellular processes such as cell growth, proliferation, differentiation, and survival. MEK acts as an intermediary between upstream signaling molecules and downstream effectors, ultimately leading to the activation of MAPKs. These MAPKs then initiate a cascade of events that regulate gene expression and cellular responses. Dysregulation of MEK signaling has been implicated in various diseases, including cancer, making it an important target for therapeutic interventions in the pharmaceutical industry.
MEK-targeted drugs can play the following three roles:
1. Inhibition of tumor cell proliferation. MEK-targeted drugs inhibit the activation of the signal transduction pathway, blocking the phosphorylation of downstream ERK protein kinases and their stimulation of gene transcription and protein synthesis, thereby reducing tumor cell proliferation. Moreover, MEK inhibitors can also induce tumor cell death and further inhibit tumor cell proliferation by regulating the expression of cell cycle proteins and apoptosis-related proteins.
2. Inhibition of tumor cell invasion and metastasis. Some studies have shown that MEK-targeted drugs can inhibit cell movement and attachment to the extracellular matrix, hence hindering the invasion and metastasis of tumor cells.
3. Targeting gene mutations in tumor cells. In certain types of cancer, such as melanoma and colon cancer, the efficacy of MEK-targeted drugs may be related to the patient's gene mutations. For instance, in melanoma, MEK-targeted drugs can exert their effect by inhibiting the overactivation of the RAF/MEK/ERK signaling pathway caused by BRAF gene mutations.
MEK Competitive Landscape
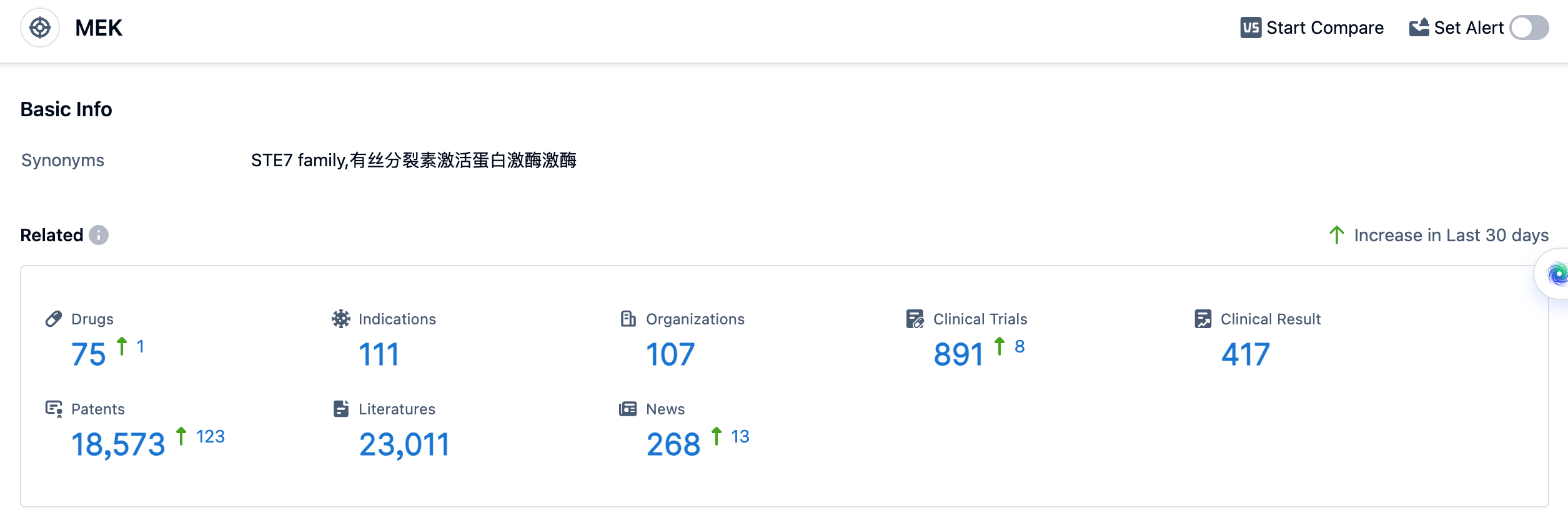
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 18 Sep 2023, there are a total of 75 MEK drugs worldwide, from 107 organizations, covering 111 indications, and conducting 891 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
The analysis of the target MEK in the pharmaceutical industry reveals a competitive landscape with multiple companies actively involved in R&D. Pfizer Inc., Roche Holding AG, AstraZeneca PLC, Novartis AG, and Merck & Co., Inc. are the companies with the highest number of drugs in various development phases. Melanoma, Non-Small Cell Lung Cancer, Colorectal Cancer, and Neurofibromatosis 1 are some of the indications for which drugs have been approved. Small molecule drugs are the most common drug type progressing rapidly under the target MEK, indicating intense competition. Overall, the target MEK presents significant opportunities for the development of innovative drugs in the treatment of various cancers.
Selective MEK 1/2 Inhibitor: Selumetinib
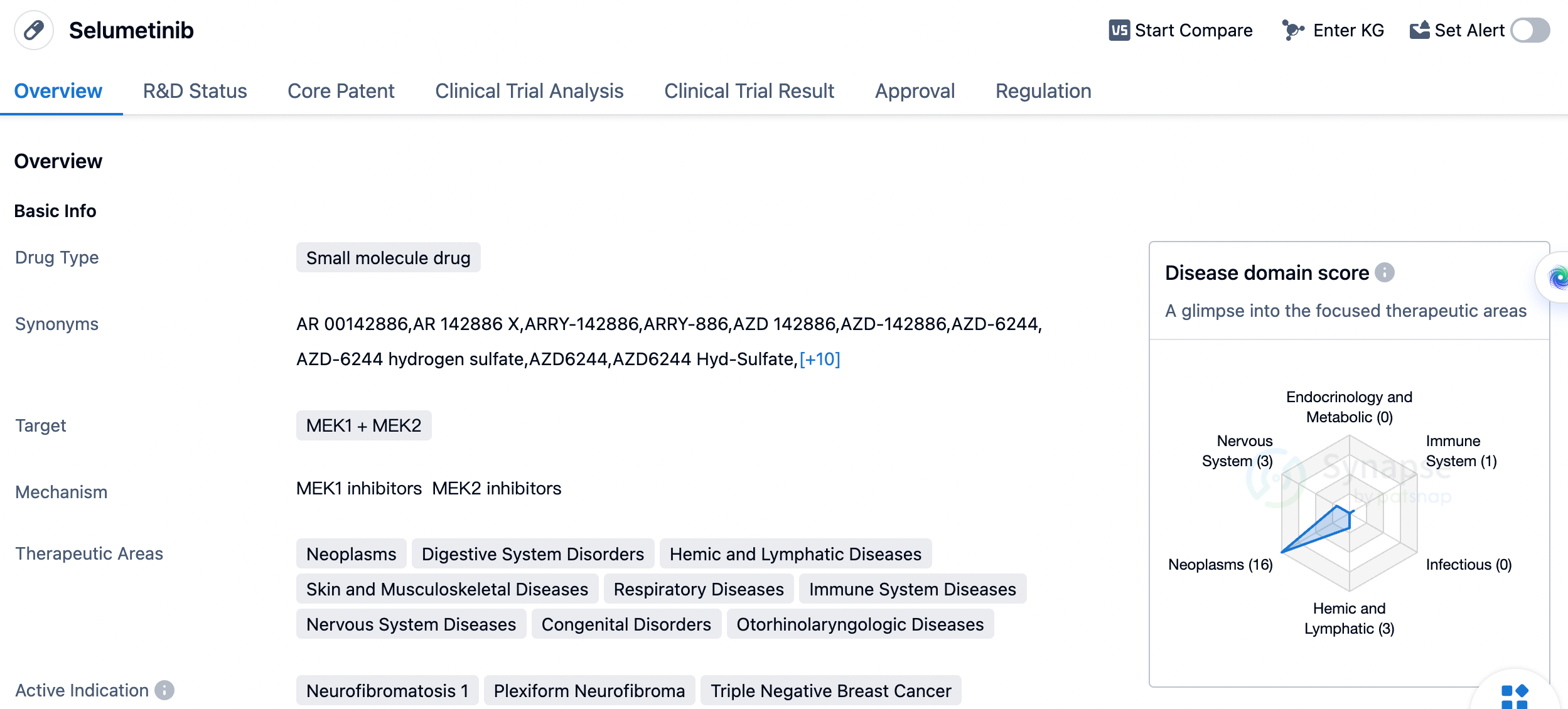
Selumetinib is an orally administered, selective MEK 1/2 inhibitor that controls abnormal cell proliferation and tumor growth by inhibiting the overactivated RAS pathway through targeting the signaling regulating enzyme MEK downstream of RAS. Neurofibromatosis type 1 (NF1) is a hereditary neurologic disease caused by NF1 gene mutations, characterized by overactive RAS signaling pathways due to NF1 gene mutations, thereby triggering abnormal cell proliferation and tumorigenesis. Plexiform neurofibromas (PN), a common benign tumor in NF1, appears in up to 50% of NF1 patients. In 2020, Selumetinib was approved by the US Food and Drug Administration (FDA) for the treatment of NF1 patients aged 2 and above with symptomatic, inoperable PN, making it the first innovative therapy for NF1 worldwide, and it was approved in China in May of this year.
Selumetinib was developed by Array BioPharma, Inc., and it has received approval in both the global market and China. The drug obtained its first approval in the United States in April 2020. It has undergone regulatory processes such as priority review, orphan drug designation, and breakthrough therapy designation.
In summary, Selumetinib is a small molecule drug that targets MEK1 and MEK2. It has been approved for various therapeutic areas and has shown efficacy in treating multiple active indications. Developed by Array BioPharma, Inc., the drug has received global and Chinese approvals. Its first approval was granted in the United States in April 2020, and it has undergone regulatory processes such as priority review, orphan drug designation, and breakthrough therapy designation.
Reversible Inhibitor: Binimetinib
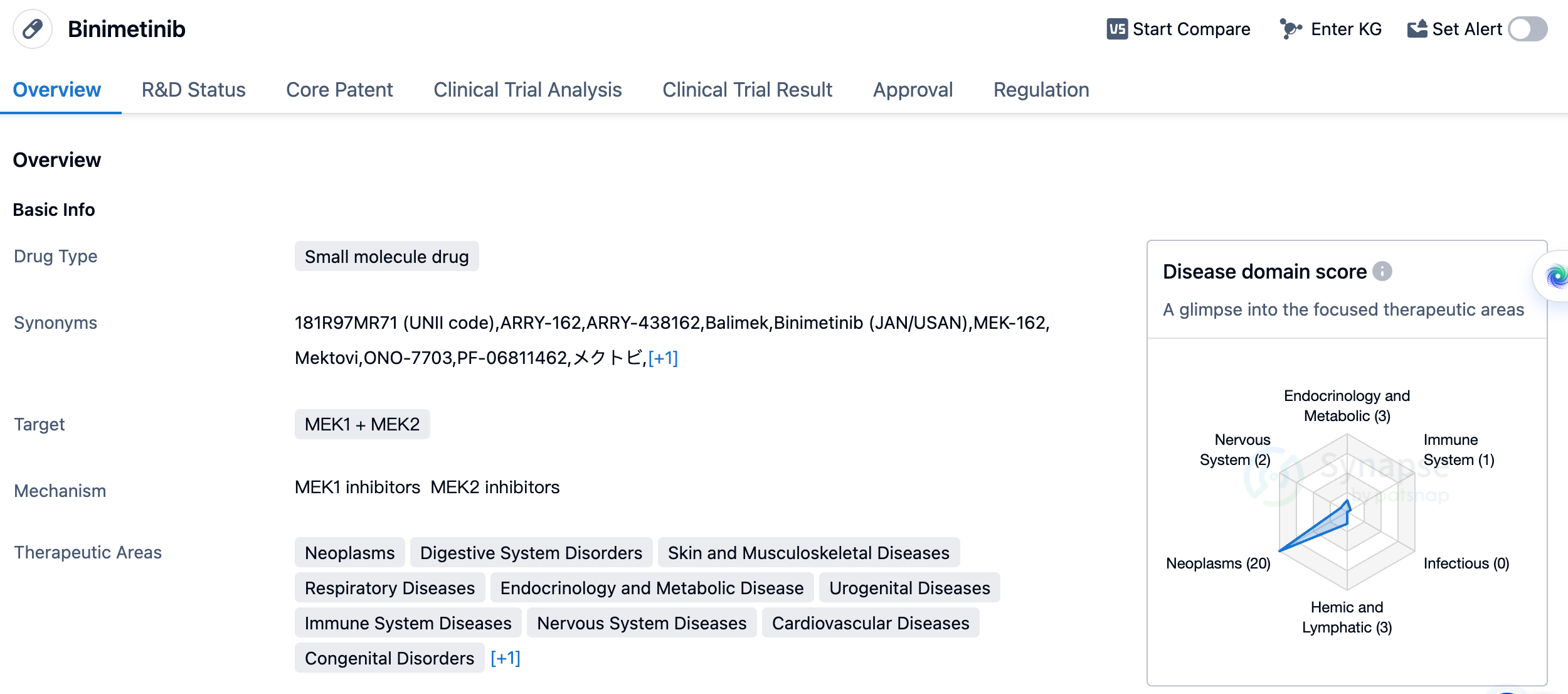
Binimetinib is a reversible inhibitor of the activity of mitogen-activated extracellular signal-regulated kinases 1 (MEK1) and MEK2. MEK proteins are upstream regulators of the extracellular signal-related kinase (ERK) pathway. In vitro, binimetinib inhibits ERK phosphorylation in cell-free immunoassays, as well as the vitality and MEK-dependent phosphorylation of BRAF-mutated human melanoma cell lines. Furthermore, Binimetinib also inhibits in vivo ERK phosphorylation and tumor growth in BRAF-mutated mouse xenograft models.
Binimetinib has been developed by Array BioPharma, Inc. and has received approval for use in various therapeutic areas. These areas include neoplasms, digestive system disorders, skin and musculoskeletal diseases, respiratory diseases, endocrinology and metabolic disease, urogenital diseases, immune system diseases, nervous system diseases, cardiovascular diseases, congenital disorders, and hemic and lymphatic diseases.
Binimetinib has been approved for use in the United States, with the first approval date recorded in June 2018. The drug has also reached phase 2 of development in China. And it is classified as an orphan drug, indicating that it is intended to treat rare diseases or conditions.
In summary, Binimetinib is a small molecule drug developed by Array BioPharma, Inc. It targets MEK1 and MEK2 and has been approved for use in various therapeutic areas, particularly in the treatment of different types of cancer. The drug has achieved its highest phase of development globally and has received approval in the United States. It is also in phase 2 of development in China. Binimetinib is classified as an orphan drug, providing regulatory advantages to its manufacturer.