Allergy Terminator: Leukotriene Receptor Antagonist
CysLT, or cysteinyl leukotrienes, are inflammatory mediators produced by various cells in the human body, including mast cells, eosinophils, and macrophages. These molecules play a crucial role in the pathophysiology of asthma and allergic diseases. CysLTs bind to specific receptors, such as CysLT1 and CysLT2, leading to bronchoconstriction, increased mucus production, and recruitment of inflammatory cells. This results in airway inflammation, narrowing of the airways, and subsequent respiratory symptoms. Understanding the role of CysLT in the human body has paved the way for the development of targeted therapies, such as leukotriene receptor antagonists, which effectively alleviate symptoms and improve the quality of life for individuals with asthma and allergic conditions.
Leukotriene antagonists work by blocking the action of leukotrienes, they are mainly used for preventing asthma, controlling allergic rhinitis or allergic reactions. Leukotriene receptor antagonists are a class of non-steroidal anti-inflammatory drugs, which mainly function by competitively binding to cysteinyl leukotriene (CysLTs) receptors and blocking the activity of CysLTs, such as improving increased vascular endothelial permeability, excessive mucus secretion, bronchial smooth muscle contraction, respiratory tissue fibrosis and remodeling, etc.
Leukotriene Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 18 Sep 2023, there are a total of 45 CysLT drugs worldwide, from 41 organizations, covering 21 indications, and conducting 537 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
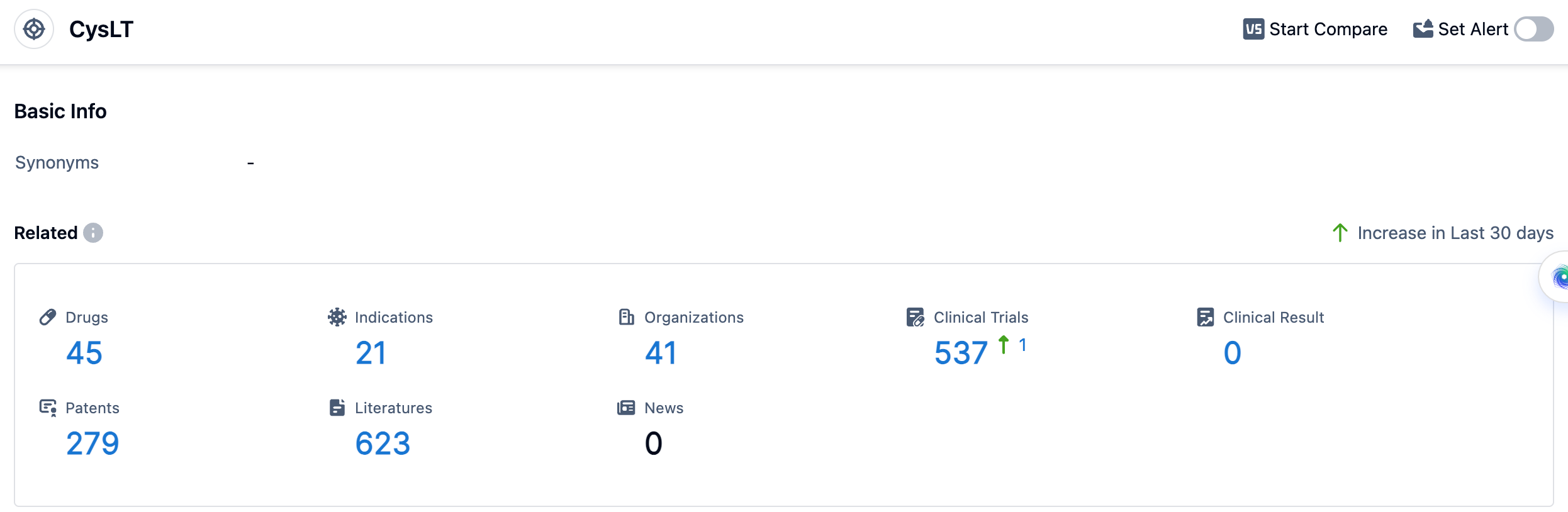 The current competitive landscape for the target CysLT shows that Merck & Co., Inc., Organon & Co., and KYORIN Pharmaceutical Co., Ltd. are leading in terms of R&D progress and approved drugs. The indications for drugs under the target CysLT are diverse, with asthma, rhinitis, and hypersensitivity being the most common indications. Small molecule drugs are progressing rapidly, indicating intense competition in the market. The United States, Japan, China, and other countries are actively developing drugs for the target CysLT. Overall, the future development of target CysLT looks promising with ongoing research and development efforts by various companies and countries.
The current competitive landscape for the target CysLT shows that Merck & Co., Inc., Organon & Co., and KYORIN Pharmaceutical Co., Ltd. are leading in terms of R&D progress and approved drugs. The indications for drugs under the target CysLT are diverse, with asthma, rhinitis, and hypersensitivity being the most common indications. Small molecule drugs are progressing rapidly, indicating intense competition in the market. The United States, Japan, China, and other countries are actively developing drugs for the target CysLT. Overall, the future development of target CysLT looks promising with ongoing research and development efforts by various companies and countries.
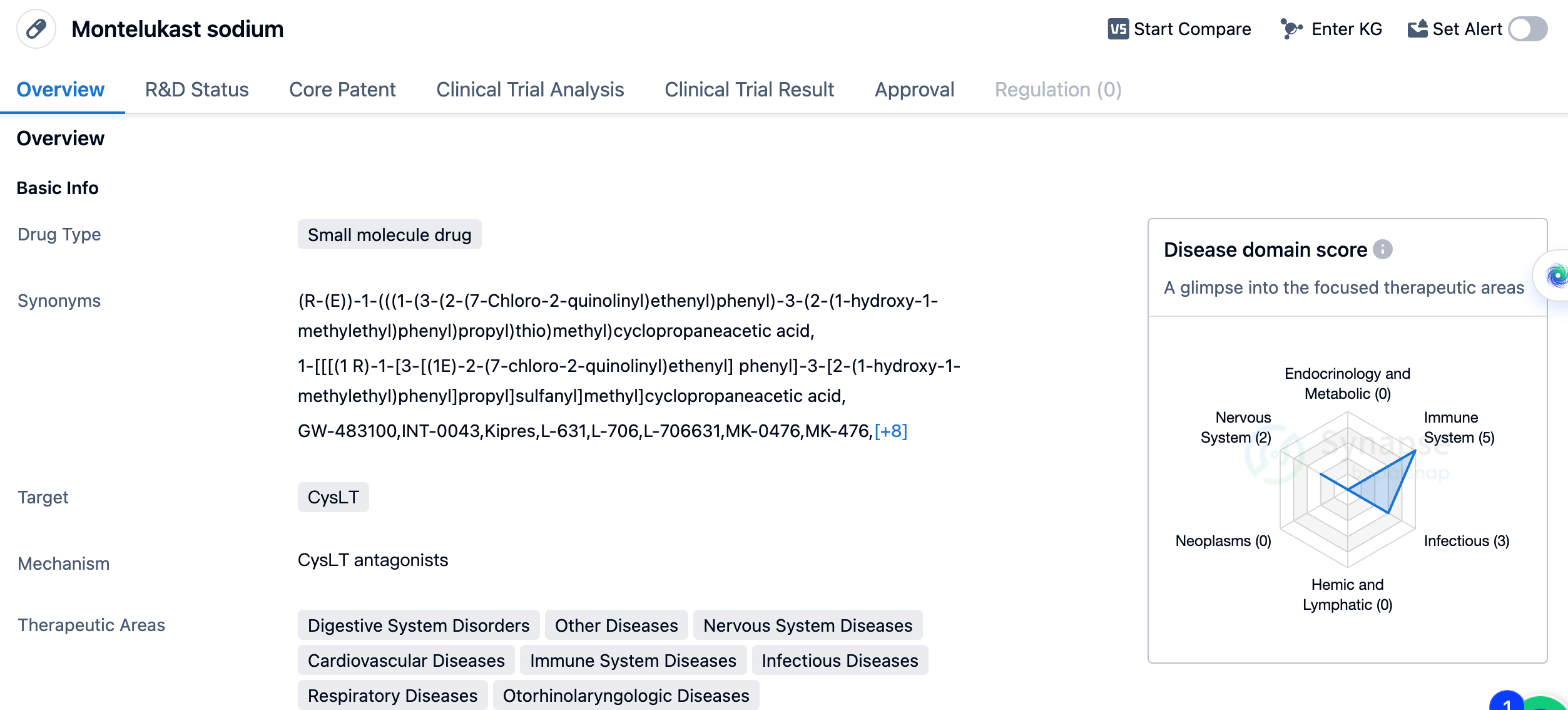
The preferred choice for pediatric asthma is Montelukast Sodium
Montelukast sodium was developed by Merck & Co., Inc., a renowned pharmaceutical company. It received its first approval in Mexico in July 1997, making it available for use in that country. Since then, it has also obtained approvals in other countries, indicating its global acceptance.
As the only long-acting leukotriene inhibitor that both children and adults can use at present. Leukotriene is an inflammatory mediator, which has a strong bronchoconstrictive and pro-inflammatory effect, and is one of the "culprits" of respiratory inflammation. Montelukast Sodium works by seizing leukotriene receptors, making leukotrienes incapable of causing trouble, thereby reducing the inflammatory response in the respiratory tract. It is suitable for the prevention and long-term treatment of asthma, alleviating the symptoms caused by allergic rhinitis, it has good tolerability, and its side effects are mild.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Overall, Montelukast sodium's approval and widespread use in multiple countries, along with its diverse therapeutic indications, make it a significant drug in the field of biomedicine. Its success can be attributed to the efforts of Merck & Co., Inc. in developing and bringing this drug to market, ultimately benefiting patients suffering from a range of conditions.
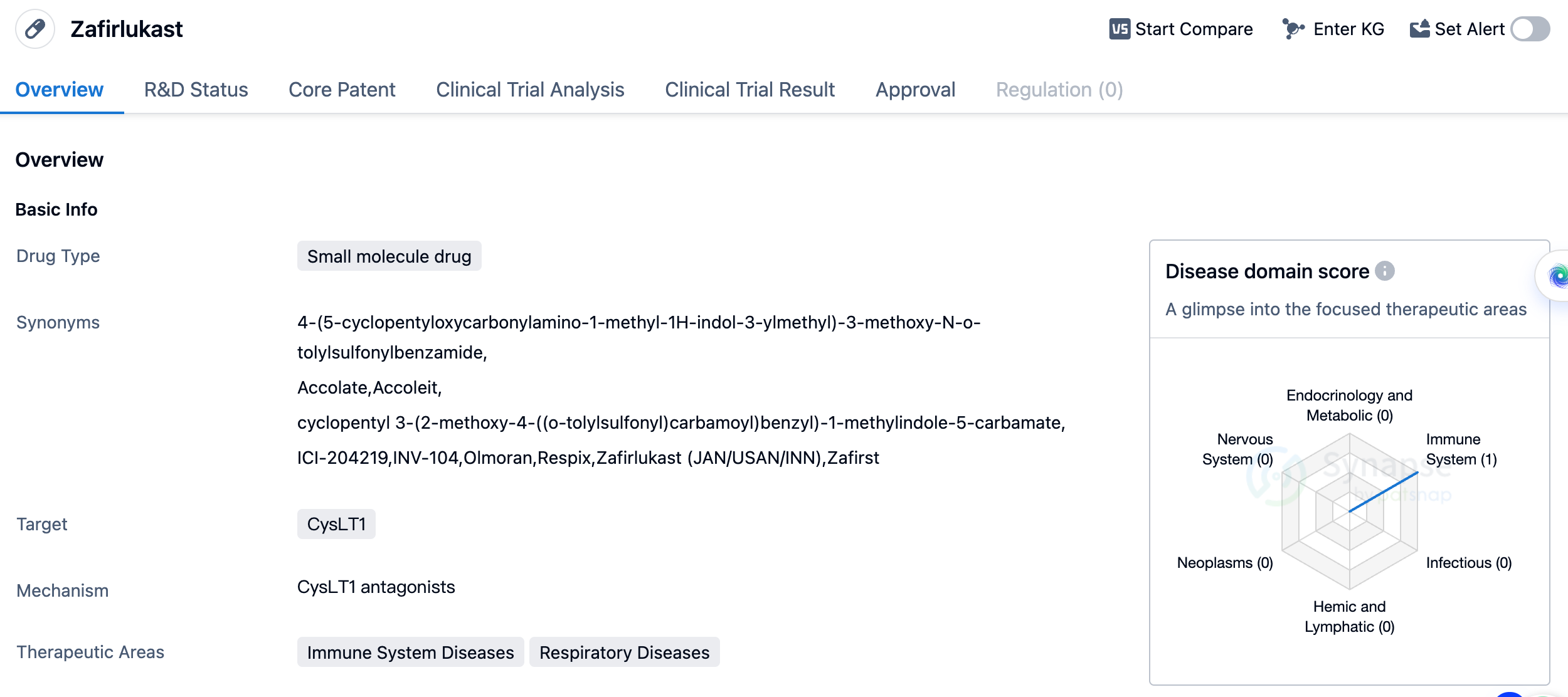
Zafirlukast
Zafirlukast, commercially known as Accolate, is developed by AstraZeneca. It is the second leukotriene receptor antagonist to come to market following Montelukast. It can significantly reduce the rapid and late-phase bronchoconstriction induced by allergens, and inhibit the increase of histamine activity induced by allergens. It is effective for mild to moderate asthma, can cause dose-dependent improvement in lung function, and can reduce the dose of β-agonists. It is used for the treatment of chronic persistent asthma in individuals aged 12 years and older. Adverse reactions: It may cause severe liver and kidney dysfunction, and interact with other medications such as warfarin. Skin rashes, urticaria and vascular edema (rarely), mild limb swelling (rarely), bleeding disorders after bruising, and granulocytopenia may occur. These events usually return to normal after stopping the medication.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
As a small molecule drug, Zafirlukast is designed to interact with specific molecular targets in the body, in this case, the CysLT1 receptor. By targeting this receptor, the drug aims to modulate the immune response and reduce inflammation, providing relief for patients suffering from asthma.
Asthma is a chronic respiratory condition characterized by inflammation and narrowing of the airways, leading to symptoms such as wheezing, shortness of breath, and coughing. Zafirlukast offers a targeted approach to managing asthma by specifically addressing the underlying immune system dysfunction associated with the disease.
Overall, Zafirlukast is an approved small molecule drug developed by AstraZeneca PLC for the treatment of asthma. Its targeting of the CysLT1 receptor makes it a valuable therapeutic option for patients with immune system and respiratory diseases. With its long history of global and Chinese approval, Zafirlukast has established itself as a trusted and effective medication in the field of biomedicine.