LSD1 inhibitors - A potent inhibitory target for tumor immunotherapy
The catalytic function of LSD1(KDM1A)depends on the flavin adenine dinucleotide (FAD) and it is a member of the monoamine oxidase family. LSD1 consists primarily of three parts: the N-terminal SWIRM domain; the C-terminal amine oxidase (AOL) domain, which is divided into the FAD-binding domain and the substrate-binding domain, forming the catalytic activity center; and the centrally located Tower domain. The Tower domain has two anti-balanced α-helices, playing a crucial role in LSD1's activity. LSD1 can regulate the expression of tumor-associated genes, promoting the proliferation, invasion, and metastasis of tumor cells. Current research has proven that LSD1 plays a critically regulatory role in hematological malignancies, such as acute granulocytic leukemia. LSD1 has also been found to show abnormal expression in a variety of solid tumors, closely related to tumor tissue grading, malignancy degree, and prognosis.
LSD1, also known as lysine-specific demethylase 1A, is an enzyme that plays a crucial role in the regulation of gene expression in the human body. It functions by removing specific methyl groups from histone proteins, which are involved in the packaging of DNA. By demethylating histones, LSD1 helps to activate or repress gene transcription, thereby influencing various cellular processes such as cell differentiation, development, and response to environmental stimuli. Dysregulation of LSD1 has been implicated in several diseases, including cancer and neurodegenerative disorders. Understanding the role of LSD1 can provide valuable insights for the development of targeted therapies in the pharmaceutical industry.
LSD1 Competitive Landscape
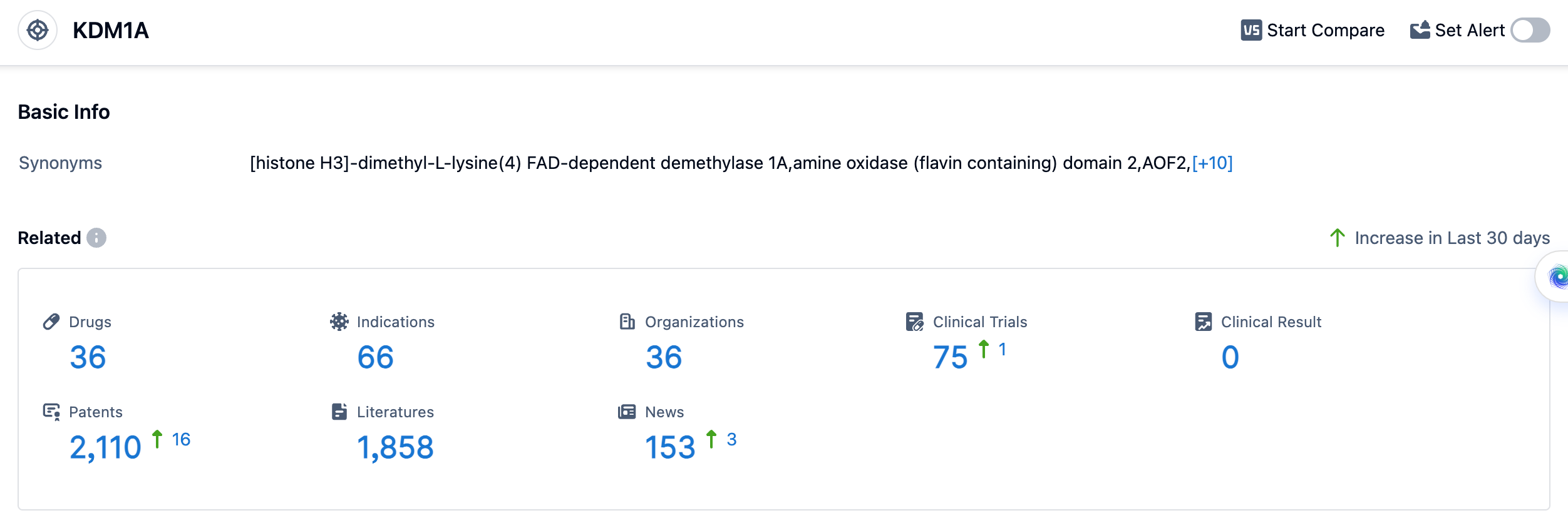
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 18 Sep 2023, there are a total of 36 LSD1 drugs worldwide, from 36 organizations, covering 66 indications, and conducting 75 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
The analysis of the target LSD1 reveals a competitive landscape with multiple companies actively developing drugs at different stages of development. Oryzon Genomics SA stands out with the highest stage of development and multiple drugs in different stages. The indications for the target LSD1 cover a wide range of diseases, with Phase 2 being the highest phase of development. Small molecule drugs are progressing rapidly, indicating a focus on developing innovative therapies. China, the United States, and the European Union are leading in terms of development, with other countries/locations also actively contributing to the research and development of LSD1-targeted therapies. Overall, the target LSD1 shows promising potential for future development in the pharmaceutical industry.
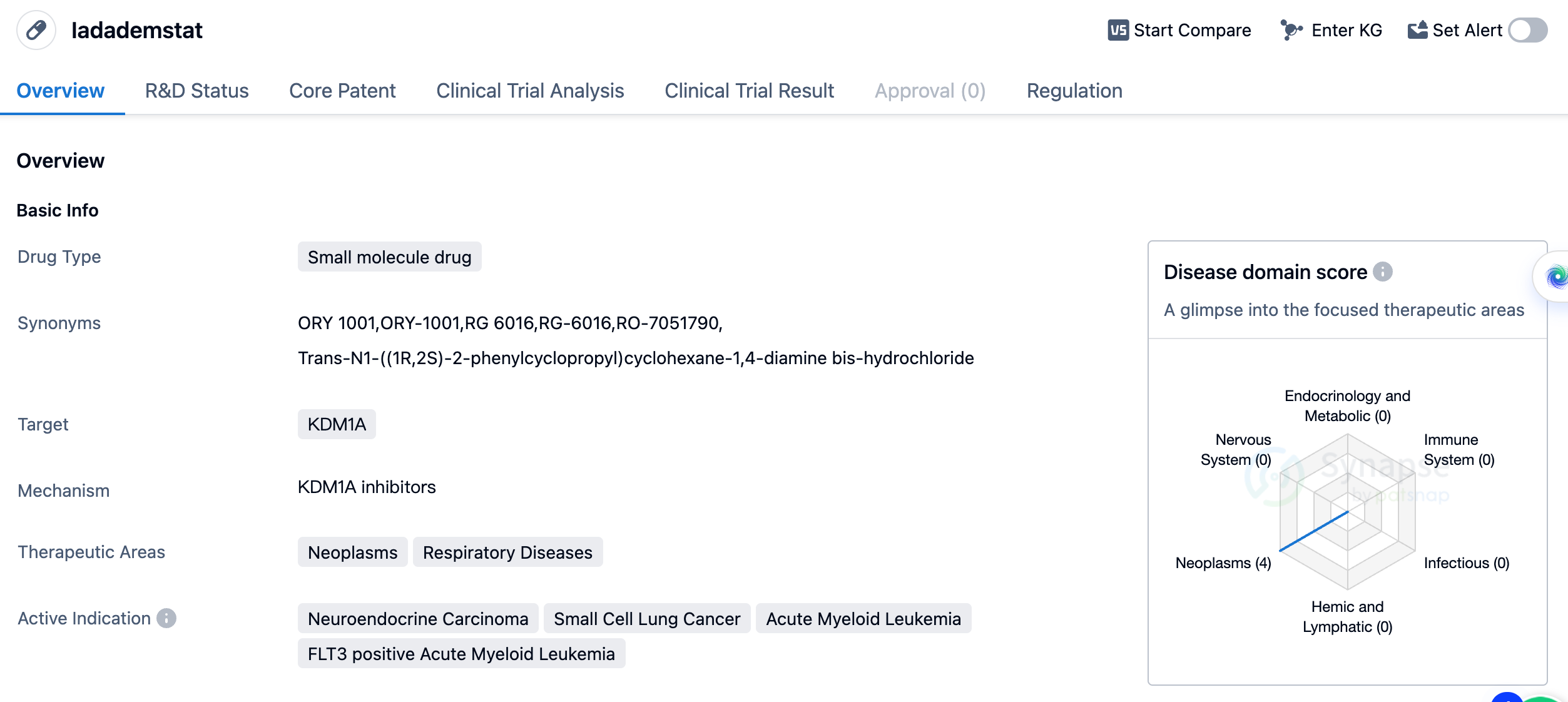
Irreversible LSD1 Inhibitor - Ladademstat
Ladademstat is a small molecule drug targeting KDM1A developed by Oryzon company. The AML trial (ALICE) data announced at the 2022 EHA showed that the overall response rate (ORR) reached 81% in 36 patients when Ladademstat was combined with azacitidine, and 64% of the CR/CRi responses were lasting (more than 6 months). By the end of the cycle, 91% of the patients had a response. In terms of safety, thrombocytopenia was observed in about half of the patients (53%), with one patient experiencing a serious adverse event. No other significant non-hematological toxicity or organ-related toxicity were observed.
In terms of therapeutic areas, Iadademstat is primarily being studied for its efficacy in treating neuroendocrine carcinoma, small cell lung cancer, acute myeloid leukemia (AML), and FLT3 positive AML. These indications represent a range of malignancies affecting different organs and systems in the body. Currently, Iadademstat has reached the highest phase of clinical development, which is Phase 2. Phase 2 trials aim to further evaluate the drug's effectiveness and side effects in a larger patient population.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In terms of regulatory status, Iadademstat has been designated as an orphan drug. Orphan drug status is granted to drugs that are intended to treat rare diseases or conditions, providing incentives to pharmaceutical companies to develop therapies for these unmet medical needs.
Based on the available information, Iadademstat shows promise as a potential treatment option for various types of cancer, including neuroendocrine carcinoma, small cell lung cancer, and AML. However, further clinical trials and research are needed to fully assess its safety and efficacy. The orphan drug designation suggests that Iadademstat may address unmet medical needs in these specific indications.
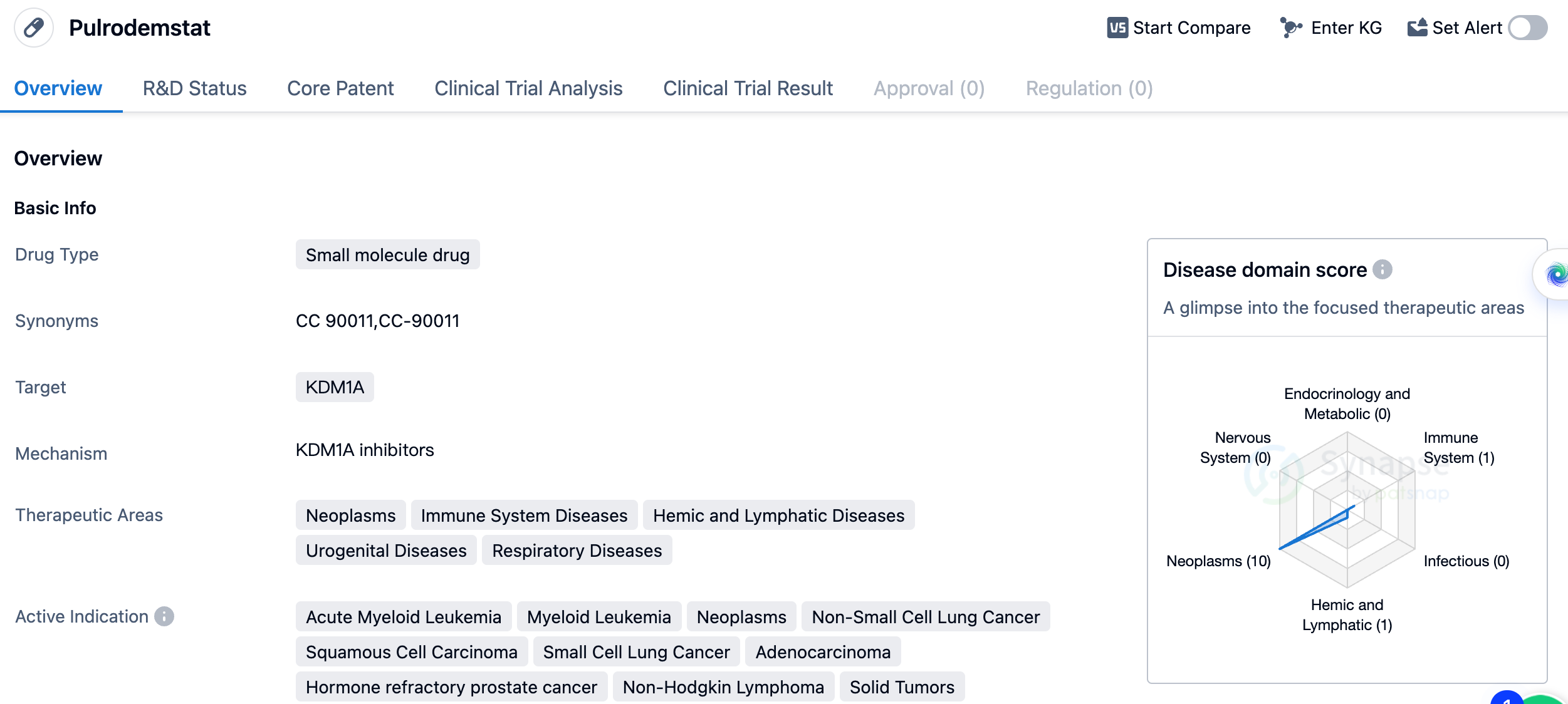
Reversible LSD1 Inhibitor: Pulrodemstat
Pulrodemstat (CC-90011) is the first reversible LSD1 inhibitor to enter clinical research, with an IC50 for LSD1 of 0.25 nmol·L−1. A phase I/II clinical trial was conducted with CC-90011 to evaluate the safety, tolerability, and efficacy of CC-90011 in conjunction with traditional AML treatments (venetoclax and azacytidine). The study showed that the use of CC-90011 with traditional AML treatments can inhibit LSD1 activity and increase the sensitivity of AML patients to traditional treatments. CC-90011-ST-001 is a phase I, multicentre, dose-escalation study, exploring nine dosage levels of CC-90011 (1.25-120mg). The primary objectives are to determine the safety, maximum tolerated dose (MTD), and/or recommended phase II dose (RP2D), with secondary objectives of assessing preliminary efficacy and pharmacodynamics.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In the field of oncology, Pulrodemstat has shown promise in treating acute myeloid leukemia, myeloid leukemia, non-small cell lung cancer, squamous cell carcinoma, small cell lung cancer, adenocarcinoma, hormone refractory prostate cancer, non-Hodgkin lymphoma, and solid tumors. These indications cover a wide range of cancer types, suggesting that Pulrodemstat may have broad applicability in the treatment of various malignancies.
As a small molecule drug, Pulrodemstat is likely to have advantages in terms of its pharmacokinetic properties, such as oral bioavailability and tissue penetration. These characteristics can contribute to its effectiveness and ease of administration.
In summary, Pulrodemstat is a small molecule drug that targets KDM1A and is being developed by Quanticel Pharmaceuticals, Inc. It has shown potential in treating various therapeutic areas, particularly in the field of oncology. With its current status in Phase 2 of clinical trials, Pulrodemstat represents a promising candidate for the treatment of a range of diseases, including acute myeloid leukemia, non-small cell lung cancer, and hormone refractory prostate cancer, among others.