Exelixis and Insilico Medicine Enter into an exclusive global licensing deal for ISM3091
Insilico Medicine and Exelixis, Inc. publicized that they're entering into a unique licensing agreement. This grants Exelixis worldwide permission to produce and market ISM3091, a potential top-tier small molecule inhibitor of USP1, identified as a synthetic lethal target correlated with BRCA-altered cancers.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Dana Aftab, Ph.D., Executive Vice President of Discovery and Translational Research, and Chief Scientific Officer at Exelixis said, "Our confident notion of the robust anti-cancer capabilities, tolerability, and pharmacokinetics of ISM3091 from preclinical trials indicates its distinctive qualities compared to other USP1 inhibitors. This marks its crucial incorporation into Exelixis’ expanding clinical-stage pipeline. As the FDA gave the green light to Insilico’s IND earlier this season, we are eager to expedite the enrollment of phase 1 trial. "
In accordance with the deal, Insilico has given Exelixis an exclusive, global license to advance and market ISM3091, along with other USP1-targeting compounds. This is in return for a foreseen upfront payment to Insilico of $80 million slated for the third quarter of 2023. Insilico also reserves the right to claim future progression, commercial and sales-based milestone payments, in addition to tiered royalties based on net sales.
"ISM3091 presents a potential top-tier method for inhibiting USP1, a key oncology target with widespread relevance in BRCA-mutant tumors," expressed Dana Aftab, Ph.D., Executive Vice President of Discovery and Translational Research, and Chief Scientific Officer at Exelixis.
USP1 facilitates the repair of DNA damage by eliminating ubiquitin from a variety of substrates; this includes proteins that reinforce the replication fork. Insilico Medicine discovered a small molecule using its generative AI platform which has extensive multiparameter optimization capacities. At the American Association for Cancer Research's Annual Meeting in April 2023, Insilico revealed selected data in a poster presentation. The FDA had given the go-ahead for the initial IND for ISM3091 targeted at patients with solid tumors on April 17, 2023.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
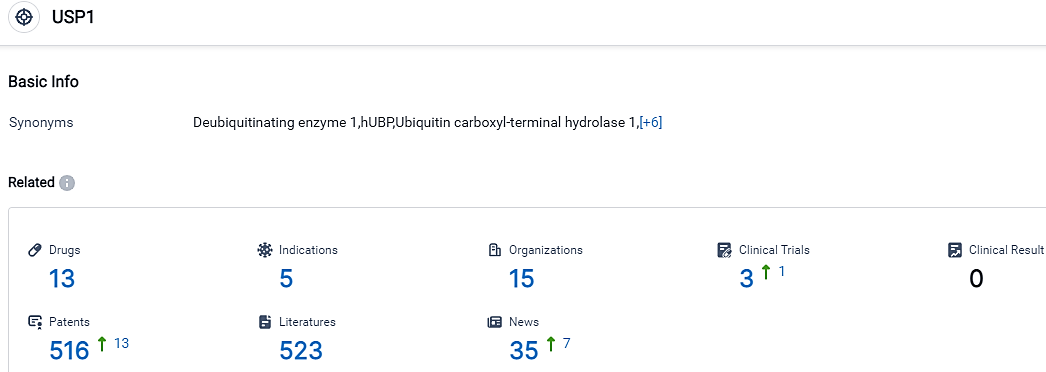 According to the data provided by the Synapse Database, As of September 17, 2023, there are 13 investigational drugs for the USP1 target, including 5 applicable indications,15 R&D institutions involved, with related clinical trials reaching 3,and as many as 516 patents.
According to the data provided by the Synapse Database, As of September 17, 2023, there are 13 investigational drugs for the USP1 target, including 5 applicable indications,15 R&D institutions involved, with related clinical trials reaching 3,and as many as 516 patents.
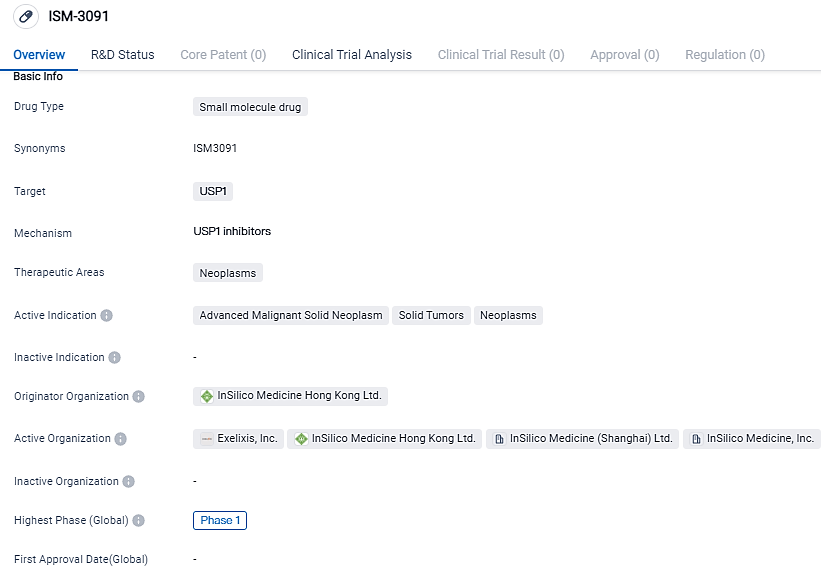
ISM-3091 is a small molecule drug being developed by InSilico Medicine Hong Kong Ltd. It targets USP1 and is primarily focused on treating advanced malignant solid neoplasms, solid tumors, and neoplasms in general. The drug is currently in Phase 1 of clinical development and has received IND approval in China. This information suggests that ISM-3091 has the potential to address a significant unmet medical need in the field of oncology.