Progress in the Study of HIV-1 Integrase Inhibitors
HIV-1 integrase is an enzyme that plays a crucial role in the replication of the human immunodeficiency virus (HIV-1) within the human body. It is responsible for the integration of viral DNA into the host cell's genome, allowing the virus to establish a persistent infection. By catalyzing the integration process, HIV-1 integrase enables the virus to evade the immune system and replicate efficiently. Understanding the function of this enzyme has been instrumental in the development of antiretroviral drugs that target integrase, inhibiting its activity and preventing viral replication. Inhibitors of HIV-1 integrase have revolutionized the treatment of HIV/AIDS, improving patient outcomes and reducing the transmission of the virus.
HIV-1 integrase Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 18 Sep 2023, there are a total of 62 HIV-1 integrase drugs worldwide, from 65 organizations, covering 14 indications, and conducting 890 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.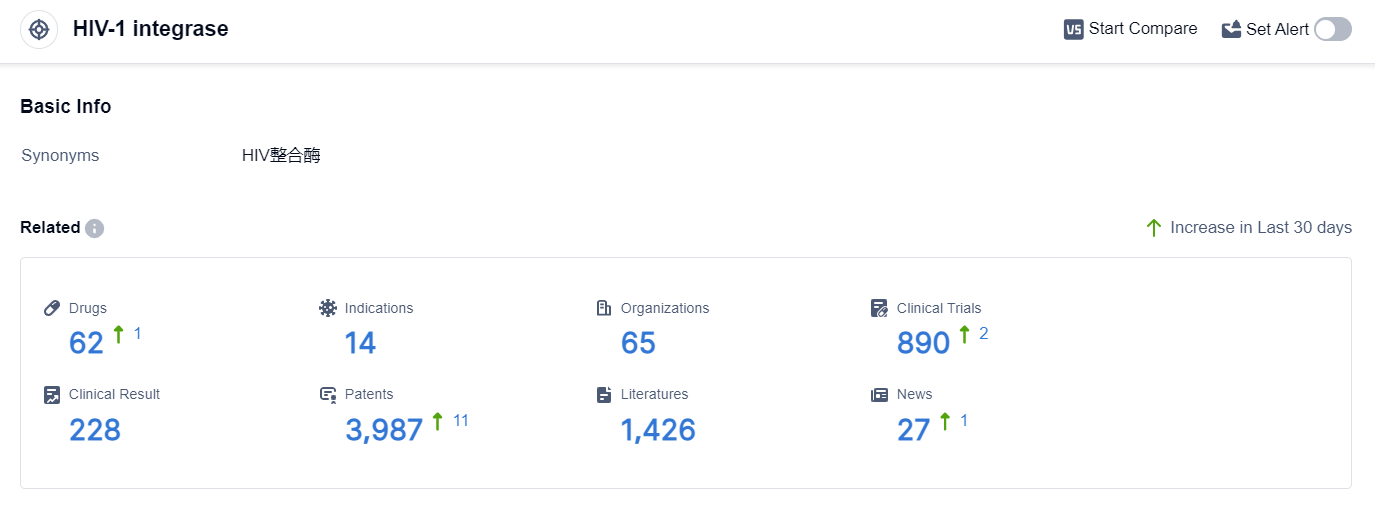
Based on the analysis of the data provided, the current competitive landscape for target HIV-1 integrase in the pharmaceutical industry is dominated by companies like GSK Plc, Gilead Sciences, Inc., and Merck KGaA. These companies have shown significant R&D progress and have multiple drugs in various stages of development.
The most common indications for approved drugs are HIV Infections, HIV-1 infection, and Acquired Immunodeficiency Syndrome. Small molecule drugs are progressing most rapidly under the current target, indicating a focus on this drug type.
The United States, European Union, and Japan are leading in terms of drug development, with China also showing progress. Overall, the target HIV-1 integrase has a promising future in terms of drug development and potential approvals for various indications.
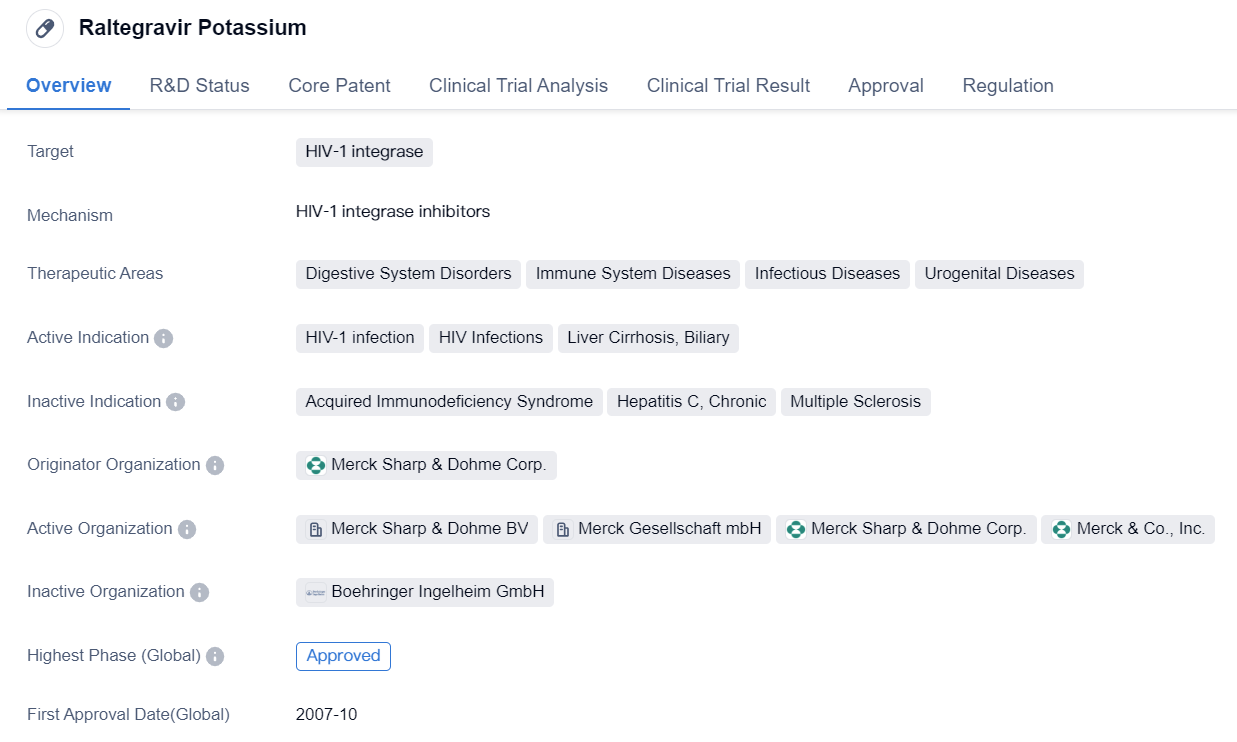
The world's first HIV-1 integrase inhibitors: Raltegravir Potassium
Raltegravir Potassium is a small molecule drug that targets HIV-1 integrase, an enzyme essential for the replication of HIV-1. It is primarily used in the treatment of HIV-1 infection and HIV infections. Additionally, it has shown potential therapeutic benefits in the management of liver cirrhosis and biliary disorders.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The drug is approved for use in various therapeutic areas, including digestive system disorders, immune system diseases, infectious diseases, and urogenital diseases. This broad range of indications highlights the versatility of Raltegravir Potassium in addressing different medical conditions.
Merck Sharp & Dohme Corp. is the originator organization responsible for the development and commercialization of Raltegravir Potassium. The drug has achieved the highest phase of approval both globally and in China, indicating its established safety and efficacy profile. It received its first approval in the United States in October 2007, making it available to patients in need of effective HIV-1 treatment.
The regulatory status of Raltegravir Potassium is classified as accelerated approval. This regulatory pathway allows for the expedited approval of drugs that address unmet medical needs, particularly in life-threatening conditions like HIV-1 infection. This designation emphasizes the urgency and importance of providing patients with access to effective treatment options.
In summary, Raltegravir Potassium is a small molecule drug developed by Merck Sharp & Dohme Corp. It specifically targets HIV-1 integrase and is approved for the treatment of HIV-1 infection and related conditions. Its therapeutic areas extend beyond HIV, encompassing digestive system disorders, immune system diseases, infectious diseases, and urogenital diseases. The drug received its first approval in the United States in 2007 and has since gained global recognition. Its accelerated approval status underscores the critical need for effective treatments in the fight against HIV-1.
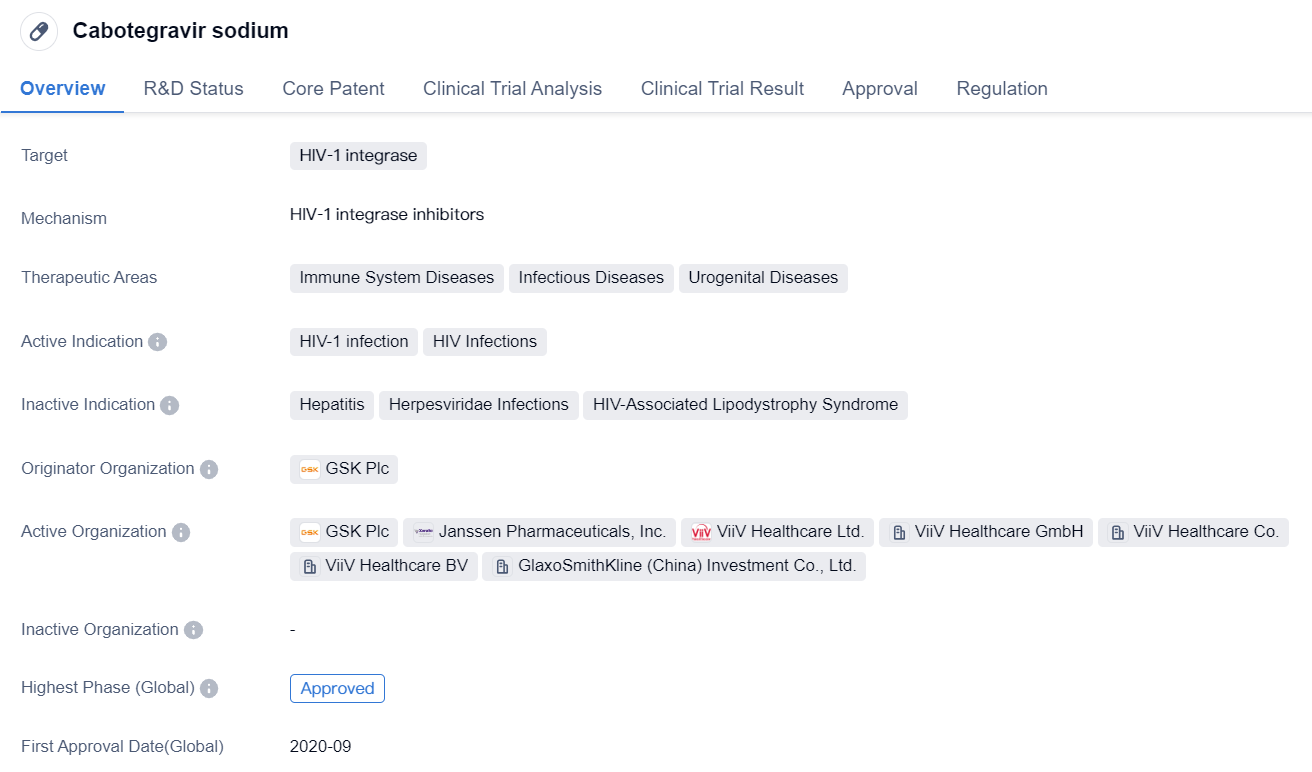
Long-acting HIV-1 integrase inhibitors: Cabotegravir sodium
Cabotegravir sodium is a small molecule drug that targets HIV-1 integrase, an enzyme essential for the replication of HIV-1. It falls under the therapeutic areas of immune system diseases, infectious diseases, and urogenital diseases. The drug is indicated for the treatment of HIV-1 infection and HIV infections.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The originator organization of Cabotegravir sodium is GSK Plc, a renowned pharmaceutical company. The drug has reached the highest phase of approval both globally and in China. It received its first approval in Canada in September 2020, making it available for use in the Canadian market.
Cabotegravir sodium has undergone various regulatory processes to expedite its development and approval. It has been granted priority review, fast track designation, breakthrough therapy status, and special review project status. These regulatory designations highlight the potential significance of the drug in addressing the unmet medical needs of patients with HIV-1 infection.
As a small molecule drug targeting HIV-1 integrase, Cabotegravir sodium offers a promising treatment option for individuals living with HIV-1 infection. By inhibiting the integrase enzyme, the drug can prevent the integration of viral DNA into the host cell's genome, thereby impeding viral replication and reducing the viral load in patients.
The approval of Cabotegravir sodium in Canada signifies a significant milestone in the fight against HIV/AIDS. It provides healthcare professionals and patients with an additional treatment option that can potentially improve patient outcomes and quality of life.
Moving forward, it will be crucial to monitor the real-world effectiveness and safety profile of Cabotegravir sodium as it becomes more widely used. Additionally, further research and clinical trials may explore the drug's potential in combination therapies or for other indications within the field of biomedicine.
In conclusion, Cabotegravir sodium is a small molecule drug developed by GSK Plc that targets HIV-1 integrase. It has received approval for the treatment of HIV-1 infection and HIV infections in Canada. The drug's regulatory designations highlight its potential as a breakthrough therapy in addressing the unmet medical needs of patients with HIV-1 infection.