Unleashing the Power of Glecaprevir/Pibrentasvir: A Comprehensive Review on R&D Breakthroughs
Glecaprevir/Pibrentasvir's R&D Progress
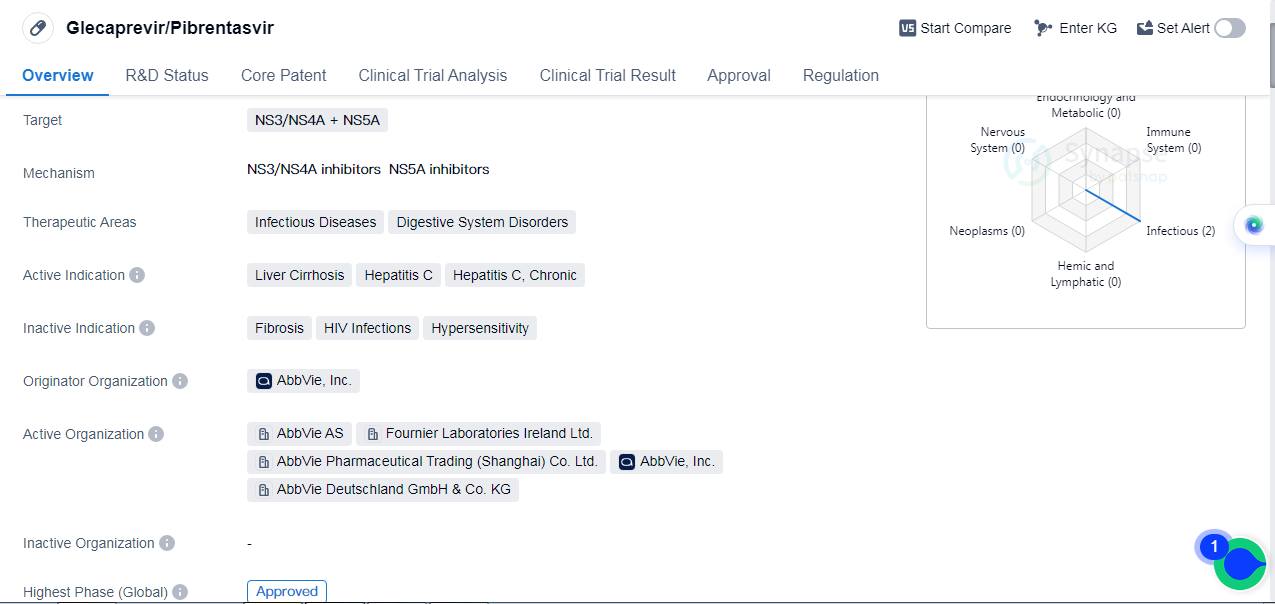
Glecaprevir/Pibrentasvir is a small molecule drug that targets NS3/NS4A and NS5A proteins. It falls under the therapeutic areas of infectious diseases and digestive system disorders. The drug is indicated for the treatment of liver cirrhosis, hepatitis C, and chronic hepatitis C.
The originator organization of Glecaprevir/Pibrentasvir is AbbVie, Inc. It has received approval for use in the global market. The drug was first approved in July 2017 by the European Union.
In terms of regulatory status, Glecaprevir/Pibrentasvir has been granted several designations. It is classified as an orphan drug, indicating that it is intended to treat rare diseases or conditions. Additionally, it has been categorized as an overseas new drug urgently needed in clinical settings, suggesting its potential to address unmet medical needs. The drug has also received breakthrough therapy designation, which is granted to drugs that show substantial improvement over existing treatments for serious conditions. Furthermore, it has undergone accelerated assessment and is on the fast track for development.
Glecaprevir/Pibrentasvir is a significant development in the field of biomedicine, particularly in the treatment of liver cirrhosis and hepatitis C. The drug's approval in multiple regions, including the European Union and China, highlights its global impact. The specific targeting of NS3/NS4A and NS5A proteins indicates its mechanism of action in combating the hepatitis C virus.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Glecaprevir/Pibrentasvir: NS3/NS4A inhibitors and NS5A inhibitors
NS3/NS4A inhibitors and NS5A inhibitors are two types of antiviral drugs used in the treatment of hepatitis C virus (HCV) infection.
From a biomedical perspective, NS3/NS4A inhibitors are a class of direct-acting antiviral agents that target the NS3 and NS4A proteins of the HCV. These proteins are essential for viral replication and assembly. By inhibiting the NS3/NS4A protease activity, these inhibitors prevent the cleavage of viral polyproteins, thereby disrupting viral replication and reducing the viral load.
NS5A inhibitors, on the other hand, target the NS5A protein of HCV. NS5A plays a crucial role in viral replication, assembly, and modulation of host immune responses. By inhibiting NS5A, these drugs interfere with viral RNA replication and assembly, leading to a decrease in viral load.
Both NS3/NS4A inhibitors and NS5A inhibitors are often used in combination with other antiviral drugs to form a direct-acting antiviral (DAA) regimen for the treatment of chronic HCV infection. These regimens have revolutionized the management of HCV by offering high cure rates and shorter treatment durations compared to older interferon-based therapies.
It is important to note that the specific NS3/NS4A inhibitors and NS5A inhibitors available in the market may vary, and the choice of drug regimen depends on factors such as the HCV genotype, patient characteristics, and treatment history.
Drug Target R&D Trends for Glecaprevir/Pibrentasvir
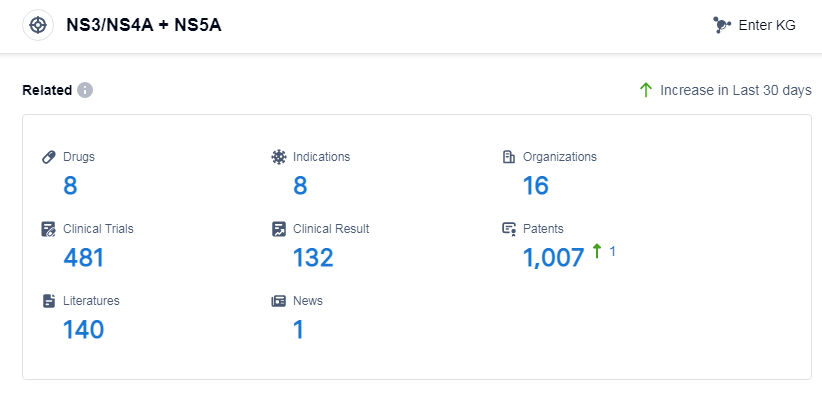
According to Patsnap Synapse, as of 7 Sep 2023, there are a total of 8 NS3/NS4A and NS5A drugs worldwide, from 16 organizations, covering 8 indications, and conducting 481 clinical trials.
The analysis of the target NS3/NS4A and NS5A reveals a competitive landscape with multiple companies actively developing drugs. AbbVie, Inc., Merck KGaA, Merck & Co., Inc., Gilead Sciences, Inc., and Bristol Myers Squibb Co. are the key players, with AbbVie, Inc. leading in terms of development stage. The most common indication targeted by these drugs is hepatitis C. Small molecule drugs are the predominant drug type, followed by synthetic peptides. The development of drugs targeting NS3/NS4A and NS5A is progressing in various countries, with China showing significant progress. Overall, the target NS3/NS4A and NS5A presents a competitive landscape with potential for future development and innovation in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Glecaprevir/Pibrentasvir represents a significant advancement in the pharmaceutical industry, particularly in the treatment of liver cirrhosis and hepatitis C. Its approval, regulatory designations, and target specificity make it a promising option for patients suffering from these conditions.