Is Rebyota approved by the FDA?
Rebyota, a fecal microbiota product, is used to prevent the recurrence of Clostridioides difficile infection (CDI). Rebyota received FDA approval on November 30, 2022. It is derived from human fecal matter donated by screened individuals and helps restore gut flora to prevent further episodes of CDI. This treatment is administered rectally 24 to 72 hours after the last dose of antibiotics for recurrent CDI. Rebyota is not intended for the initial treatment of CDI and is not studied in patients under 18 years of age.
Usage and Administration
Rebyota is administered into the rectum by a healthcare provider. Patients are typically asked to empty their bladder and bowel before the procedure. During administration, the patient is positioned on their left side or in the knee-chest position, and a water-soluble lubricant is used to minimize discomfort. The contents of the Rebyota bag are delivered by gravity through an administration tube. Patients should remain in the same position for up to 15 minutes after administration to reduce cramping.
Dosage Information
The usual adult dose for preventing recurrent CDI is 150 mL administered rectally as a single dose. This should follow antibiotic treatment for recurrent CDI.
Side Effects
Common side effects of Rebyota include:
- Stomach pain
- Diarrhea
- Bloating
- Gas
- Nausea
Patients are encouraged to report any negative side effects to the FDA.
Storage
Rebyota should be stored in an ultracold freezer (-60˚C to -90˚C or -76˚F to -130˚F). Alternatively, it can be stored in a refrigerator (2˚C to 8˚C or 36˚F to 46˚F) for up to 5 days, including thaw time. It must be completely thawed in a refrigerator for approximately 24 hours before use and should not be refrozen after thawing.
Conclusion
Rebyota offers a promising solution for preventing recurrent CDI, a condition that significantly impacts patients' quality of life. With its FDA approval in November 2022, it provides a new treatment avenue for those suffering from this persistent infection.
How to obtain the latest development progress of all drugs?
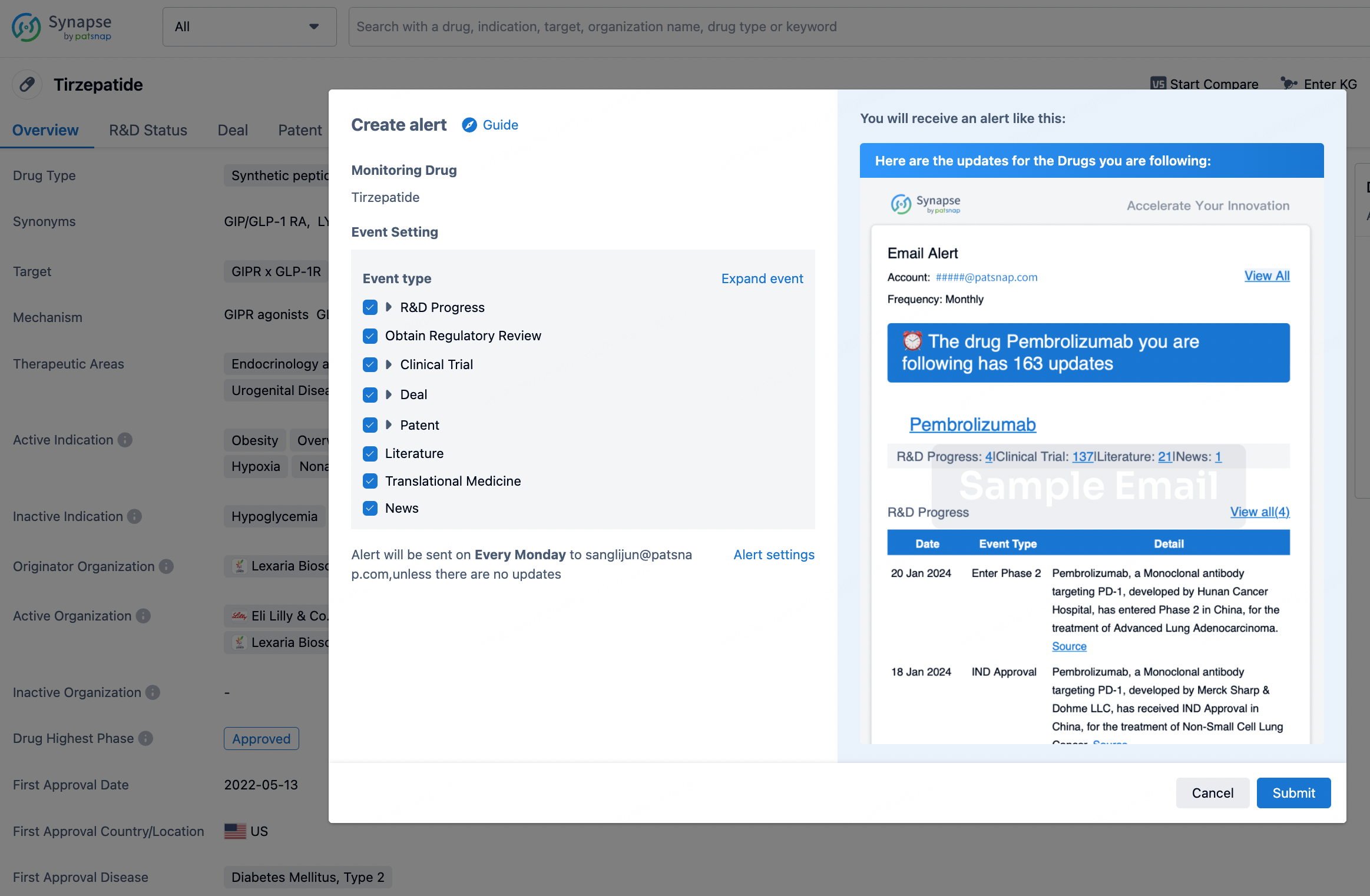
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!