IASO Bio Gains U.S. FDA Green Light for Equecabtagene Autoleucel IND Application in Multiple Sclerosis
IASO Biotechnology, a biopharmaceutical enterprise focused on the discovery, development, production, and commercialization of cutting-edge cell therapies and antibody products, has declared that its investigational new drug application for the fully human anti-BCMA chimeric antigen receptor autologous T cell injection (Equecabtagene Autoleucel, Eque-cel) has received approval from the U.S. Food and Drug Administration, specifically for the treatment of multiple sclerosis.
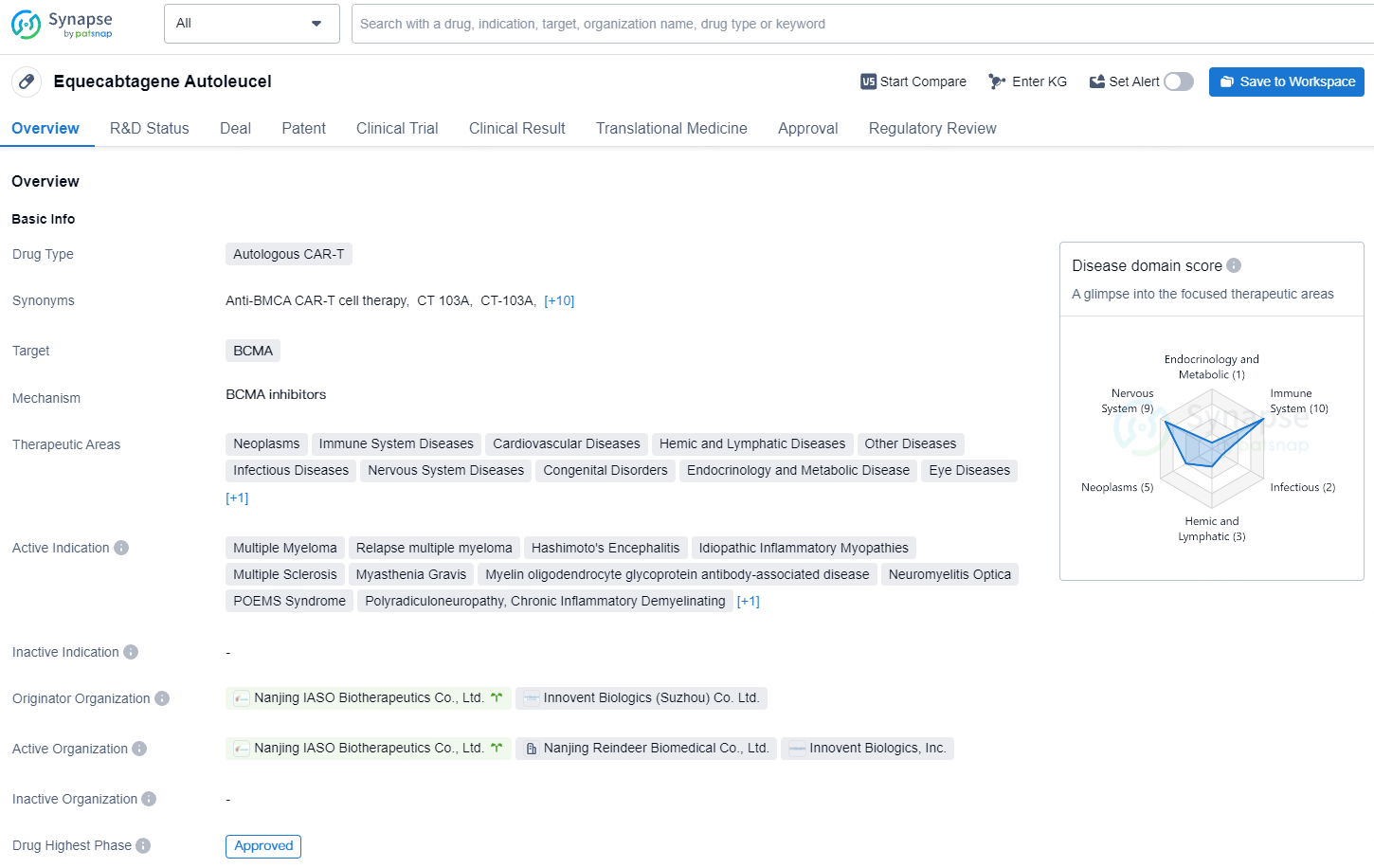
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
 In a study initiated by investigators in China, Eque-cel demonstrated encouraging efficacy across six autoimmune diseases. The IND approval for Eque-cel for MS treatment by the FDA further underscores IASO Bio’s commitment and technological progress in addressing autoimmune conditions.
In a study initiated by investigators in China, Eque-cel demonstrated encouraging efficacy across six autoimmune diseases. The IND approval for Eque-cel for MS treatment by the FDA further underscores IASO Bio’s commitment and technological progress in addressing autoimmune conditions.
Dr. Yongke Zhang, Chief Scientific Officer at IASO Bio, remarked: "Our dedication to clinical value-driven research and development remains steadfast as we aim to tackle unmet clinical needs. We are committed to implementing a global strategy and will focus on close collaboration and in-depth exchanges with international clinical research institutions. This approach is intended to expedite the development and commercialization of groundbreaking drugs, providing significant benefits to patients globally."
Multiple sclerosis (MS) is a neuroinflammatory disorder affecting the central nervous system, characterized by demyelination and neuronal damage, making it a leading cause of non-traumatic disability in young adults. Frost & Sullivan estimated that in 2023, there were about 3.07 million MS patients globally, with roughly 400 thousand in the United States. The prevalence of MS varies significantly by sex, with a female to male ratio of 3:1.
MS features focal lymphocytic infiltration in the CNS, leading to myelin destruction and axonal damage, resulting in neurological syndromes and physical disability. The clinical manifestations of MS are influenced by the location of the CNS lesions and can include sensory and visual disturbances, motor and coordination impairment, spasticity, fatigue, pain, and cognitive deficits.
About 85%-90% of MS patients experience a relapsing-remitting form, characterized by periods of symptom exacerbation followed by remission. As the disease progresses, incomplete recovery from symptoms often occurs, leading approximately 50% of patients to develop secondary progressive MS, marked by the continuous and irreversible accumulation of neurological disability.
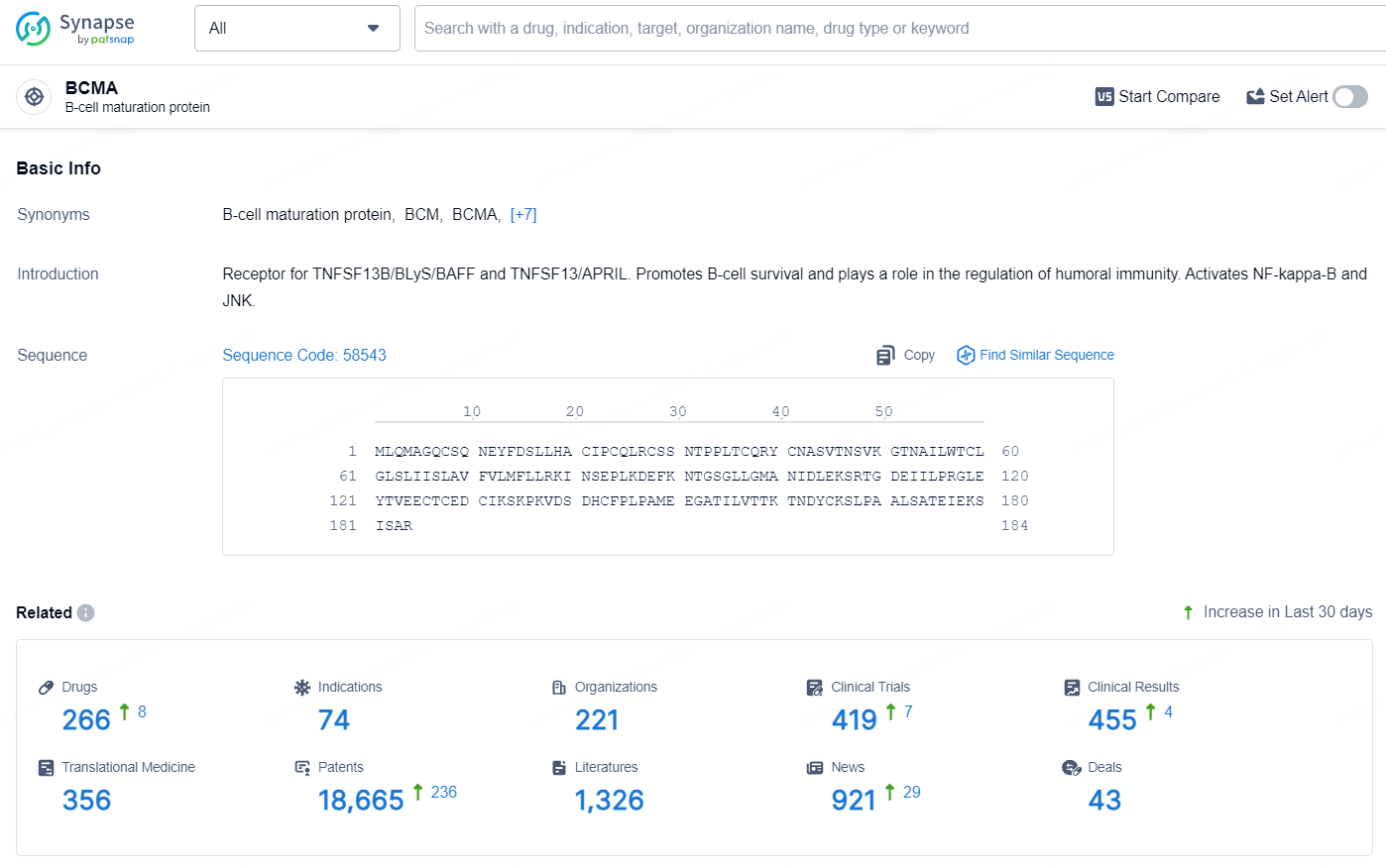
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of July 25, 2024, there are 266 investigational drugs for the BCMA target, including 74 indications, 221 R&D institutions involved, with related clinical trials reaching 419, and as many as 18665 patents.
Equecabtagene autoleucel represents a significant advancement in the field of biomedicine, particularly in the area of CAR-T therapy, and has the potential to address unmet medical needs for patients with a variety of challenging diseases across different therapeutic areas. The drug's approval and regulatory designations point to its importance and potential impact in the pharmaceutical industry. As an autologous CAR-T therapy targeting BCMA, equecabtagene autoleucel offers promising prospects for the treatment of a broad range of diseases, particularly in the field of oncology and immunology.