Vertex's ALYFTREK™ Wins US FDA Approval: New Daily Treatment for Cystic Fibrosis
Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) has announced that the U.S. Food and Drug Administration (FDA) has granted approval for ALYFTREK (vanzacaftor/tezacaftor/deutivacaftor). This innovative triple combination cystic fibrosis transmembrane conductance regulator (CFTR) modulator is designed for once-daily use and is indicated for the treatment of cystic fibrosis (CF) in individuals aged 6 and older who possess at least one F508del mutation or another CFTR gene mutation that responds to ALYFTREK. Please refer below for essential safety information, which includes a Boxed Warning.
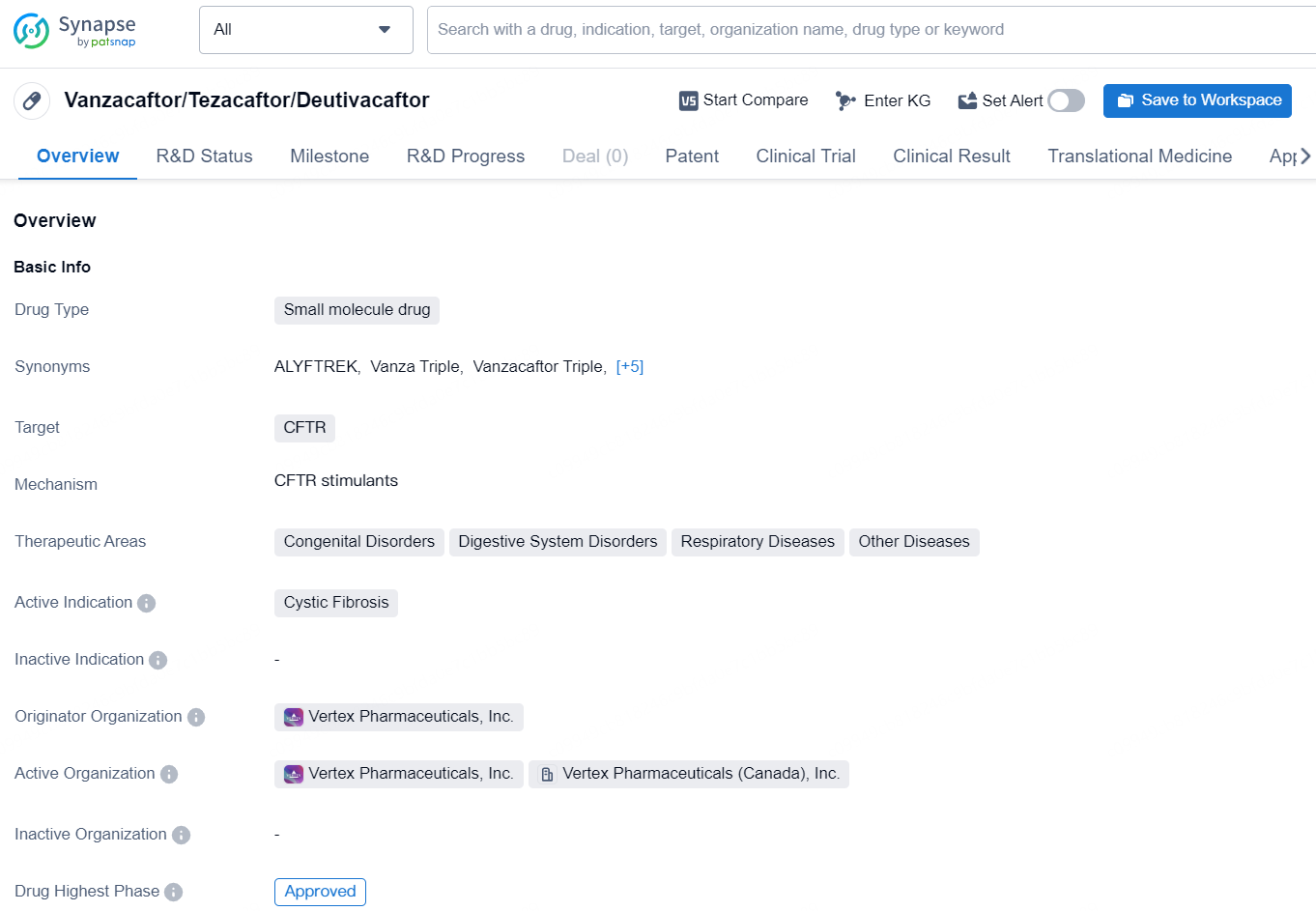
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
“ALYFTREK marks the fifth CFTR modulator to gain FDA approval, highlighting a crucial achievement in our mission to continuously innovate and enhance the quality of life for those affected by cystic fibrosis,” stated Reshma Kewalramani, M.D., CEO and President of Vertex. “For over two decades, our primary focus has been to target the root cause of cystic fibrosis, increase the number of patients treated, and help more individuals reach normal CFTR function levels. With its once-daily dosing, effectiveness against 31 additional mutations, and reduced sweat chloride levels in comparison to TRIKAFTA, ALYFTREK represents another stride toward fulfilling this objective.”
The approval is founded on the most extensive Phase 3 pivotal trial undertaken in cystic fibrosis, involving over 1,000 participants from more than 20 countries and upwards of 200 sites. Data from this research were released at the conclusion of the trials and presented at the North American Cystic Fibrosis Conference in September. The Phase 3 trials for patients with cystic fibrosis aged 12 and older achieved their main goal (proving non-inferiority in absolute change from baseline in ppFEV1 compared to TRIKAFTA) as well as all significant secondary goals, including the absolute change from baseline in sweat chloride levels. In the Phase 3 trial involving children aged 6-11 years, ALYFTREK confirmed its safety as the main endpoint, with supportive data on secondary endpoints like absolute change from baseline in ppFEV1 and sweat chloride levels, demonstrating the drug’s advantages in this younger demographic. Overall, ALYFTREK was generally well tolerated across all trials.
“In the Phase 3 clinical studies, ALYFTREK showed non-inferiority to TRIKAFTA regarding the ppFEV1 response and a significant improvement in sweat chloride levels among a diverse set of genotypes, marking a significant progress in cystic fibrosis treatment,” remarked Claire L. Keating, M.D., Co-Director of the Gunnar Esiason Adult Cystic Fibrosis and Lung Program at Columbia University and a participant in the ALYFTREK clinical trials. “ALYFTREK has the potential to enhance treatment quality for cystic fibrosis patients.”
ALYFTREK is the first CFTR modulator that can be taken once daily. A recent survey indicated that about 75% of physicians see the need for more convenient dosing as a critical unmet requirement for those with cystic fibrosis. The once-daily regimen proves to be particularly beneficial, given the necessity for patients to take CFTR modulators with meals containing fat. Additionally, ALYFTREK presents a potentially life-changing option for about 150 individuals in the U.S. with cystic fibrosis who have one of the 31 mutations and are now eligible for a CFTR modulator for the first time.
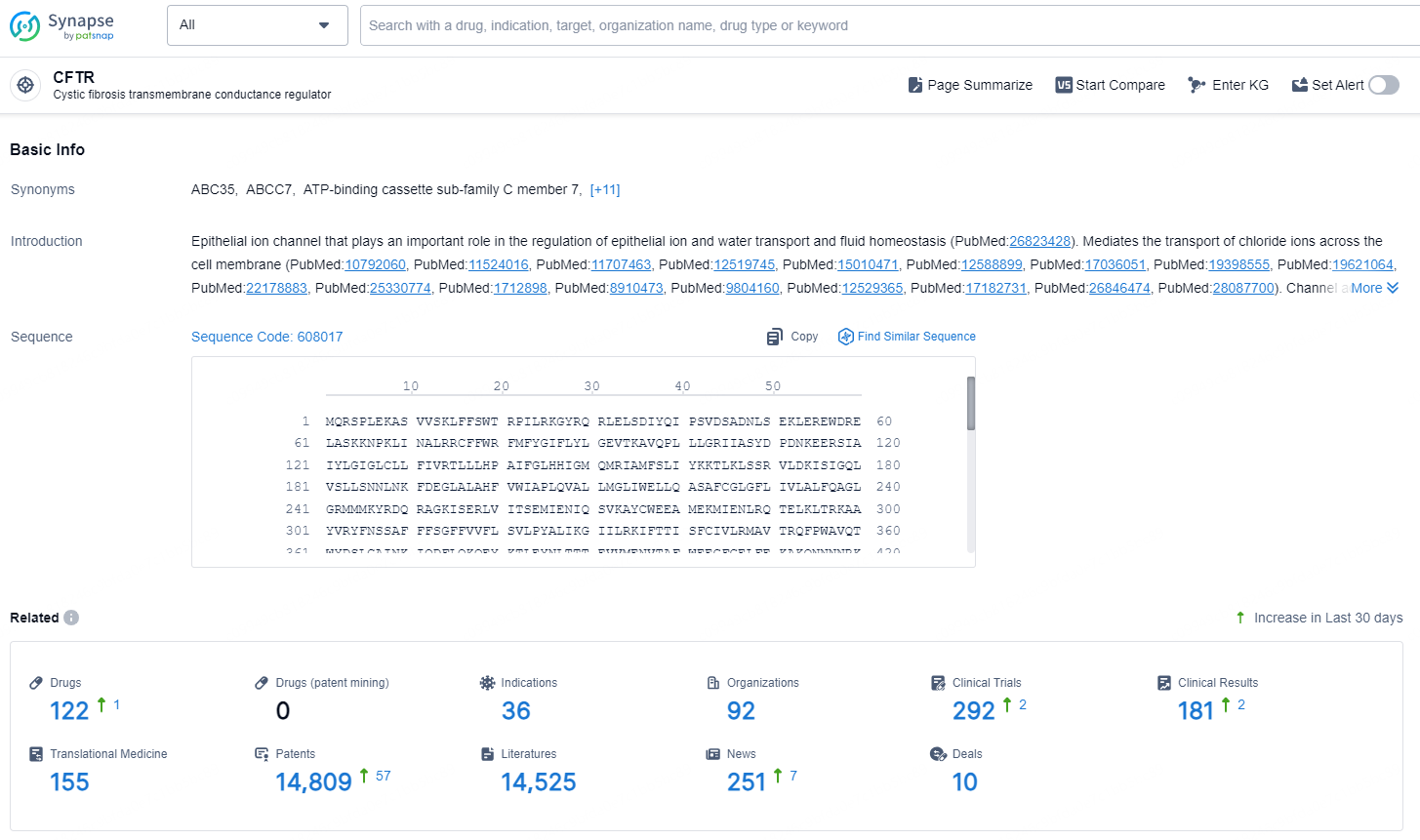
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of December 26, 2024, there are 122 investigational drugs for the CFTR target, including 36 indications, 92 R&D institutions involved, with related clinical trials reaching 292, and as many as 14809 patents.
Vanzacaftor/Tezacaftor/Deutivacaftor is a small molecule drug that targets the CFTR gene, which is associated with cystic fibrosis. Cystic fibrosis is a genetic disorder that affects the digestive and respiratory systems, and this drug is indicated for the treatment of this condition. The therapeutic areas for this drug include congenital disorders, digestive system disorders, respiratory diseases, and other diseases.