Rallybio Reveals Phase 1 Results of RLYB212 in Blood Clotting Dynamics
Rallybio Corporation (Nasdaq: RLYB), a clinical-stage biotech enterprise dedicated to translating scientific breakthroughs into groundbreaking treatments for patients afflicted with severe rare disorders, has disclosed that the Phase 1 proof-of-concept study findings of RLYB212, an innovative monoclonal antibody targeting HPA-1a under development to prevent maternal alloimmunization and fetal and neonatal alloimmune thrombocytopenia (FNAIT), have been published in the esteemed journal Thrombosis and Haemostasis. Previously, the key outcomes of this study were showcased at the prestigious 31st Congress of the International Society on Thrombosis and Haemostasis (ISTH) held in 2023.
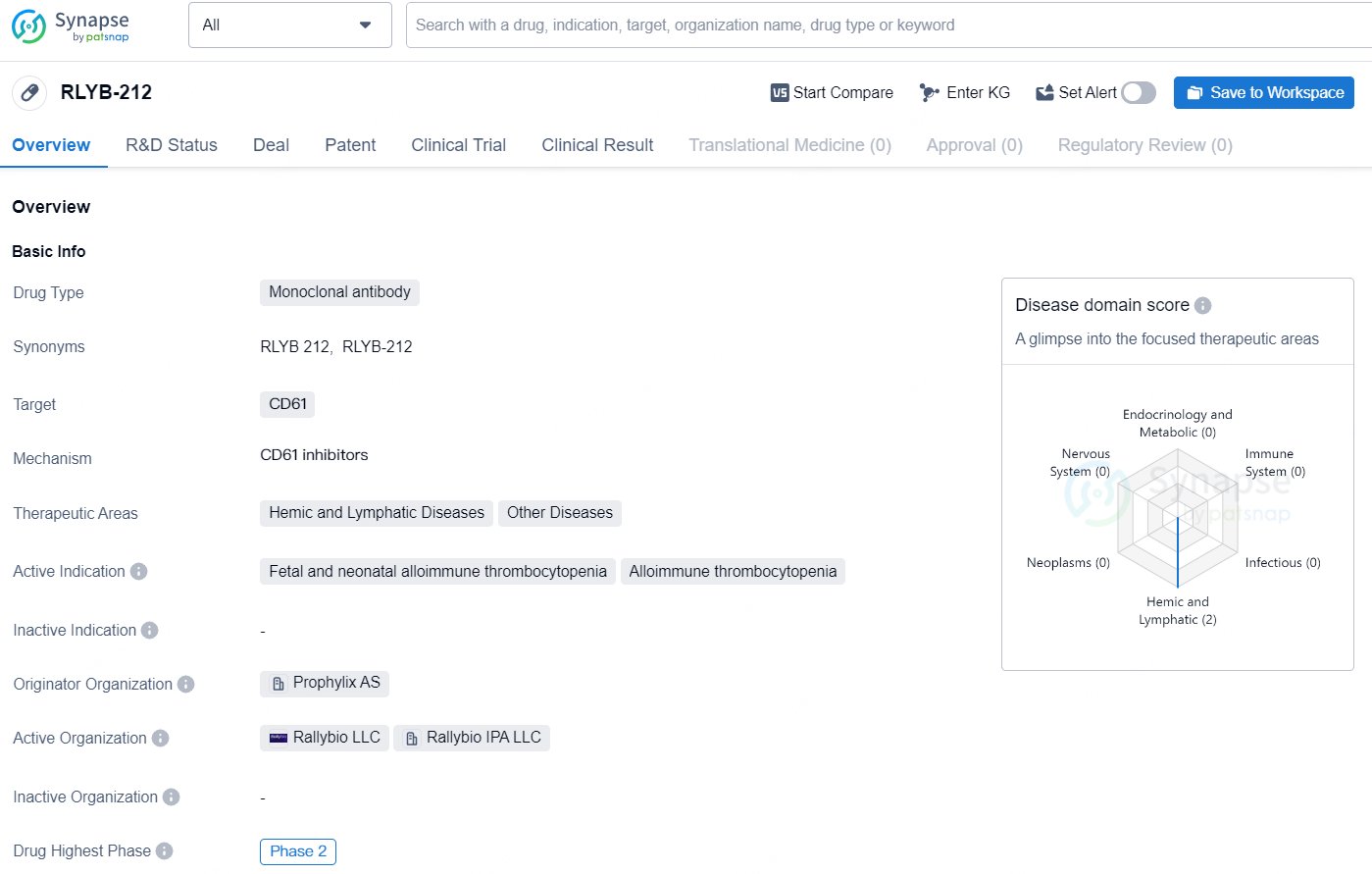
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"The Phase 1 proof-of-concept data played a pivotal role in determining the inaugural dosage for RLYB212's upcoming Phase 2 trial, scheduled to commence in Q4 2024," remarked Dr. Stephen Uden, Rallybio's CEO. "Our primary endeavor is to expedite RLYB212's development journey, aiming to introduce a novel therapeutic intervention into a critically underserved realm of maternal-fetal health."
The Phase 1 findings underscored that subcutaneous RLYB212 administration elicited a dose-responsive, swift, and total elimination of transfused HPA-1a-positive platelets in HPA-1a-negative individuals. Both dosage levels (0.09 mg and 0.29 mg) surpassed the predefined proof-of-concept threshold, achieving a ≥90% reduction in mean platelet elimination half-life compared to placebo. Coupled with extensive preclinical data, these outcomes were fundamental in establishing therapeutic exposure milestones that Rallybio believes will safely and effectively forestall maternal alloimmunization to fetal antigens, a prerequisite step leading to FNAIT.
Mimicking a substantial 30 mL fetal-maternal hemorrhage, the bolus challenge with HPA-1a-positive platelets served as a proxy. Following subcutaneous RLYB212 administration, platelet elimination patterns mirrored those observed with RhD-positive erythrocytes post-intramuscular anti-RhD administration, a well-established method to prevent RhD alloimmunization during pregnancy. In line with prior reports, RLYB212 was generally well-tolerated, without any serious or severe adverse events noted.
Rallybio remains on course to launch a Phase 2 dose-confirmation trial in pregnant women at elevated risk of HPA-1a alloimmunization and FNAIT during Q4 2024. Concurrently, the Company is actively screening pregnant women for its ongoing FNAIT natural history study, aimed at generating a contemporary dataset on HPA-1a alloimmunization frequency across a racially and ethnically diverse population. This dataset will serve as a control arm for a contemplated Phase 3 trial.
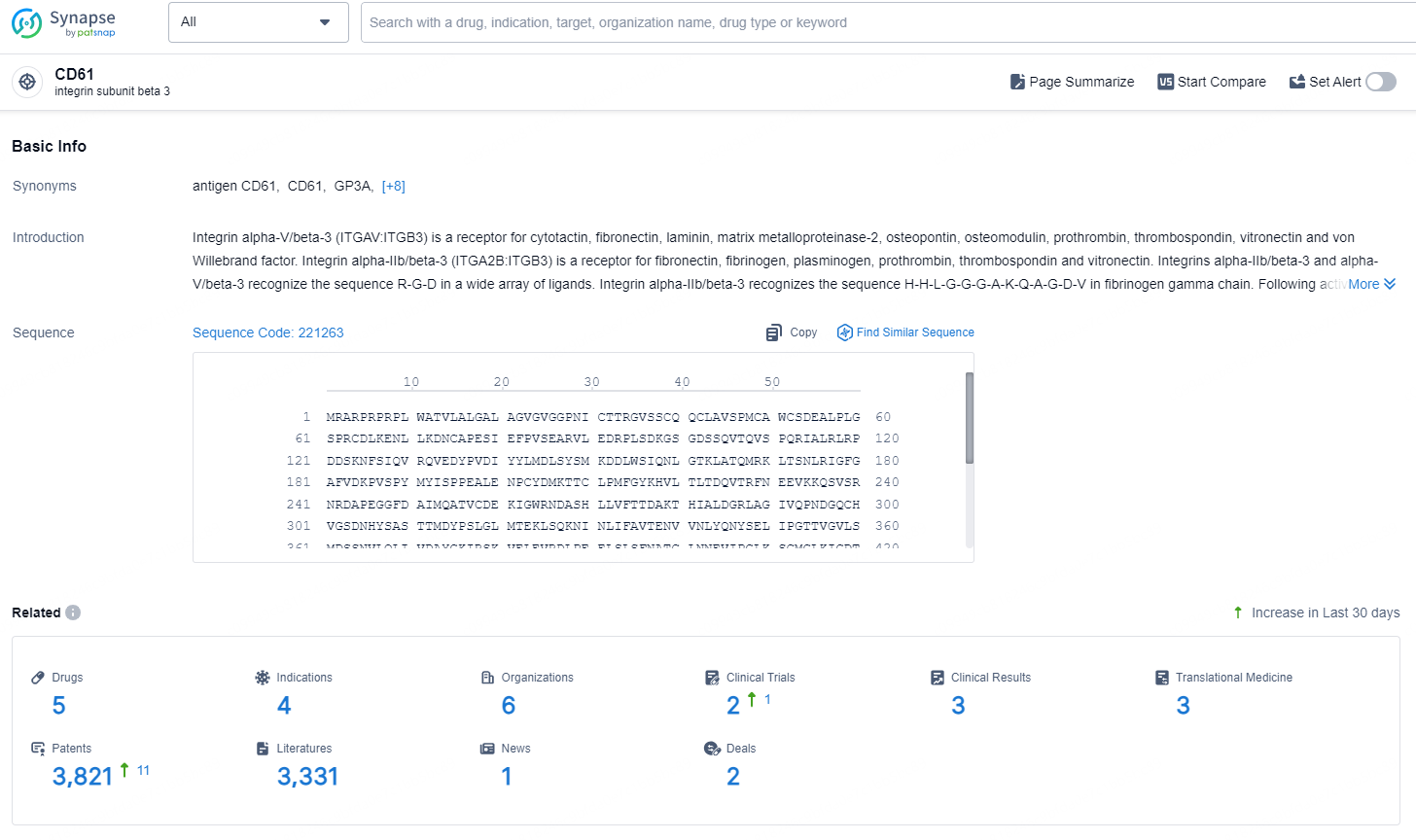
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 2, 2024, there are 5 investigational drugs for the CD61 targets, including 4 indications, 6 R&D institutions involved, with related clinical trials reaching 2, and as many as 3821 patents.
The drug RLYB-212 is a monoclonal antibody that targets CD61. It falls under the therapeutic areas of Hemic and Lymphatic Diseases, as well as Other Diseases. The active indications for this drug are Fetal and neonatal alloimmune thrombocytopenia, as well as Alloimmune thrombocytopenia. The originator organization for RLYB-212 is Prophylix AS. As of the highest phase in global development, RLYB-212 has reached Phase 2. This indicates that the drug has shown promise in early clinical trials and is moving towards further testing and potential approval. Phase 2 trials involve a larger group of patients than Phase 1 trials, and are designed to evaluate the effectiveness and safety of the drug for specific indications.