Regenxbio reports positive Phase II interim results for AAVIATE® with ABBV-RGX-314 administered via sclera to treat wet AMD
REGENXBIO Inc. unveiled encouraging preliminary findings from its Phase II AAVIATE® study involving ABBV-RGX-314. This trial focuses on exploring the efficacy of suprachoroidal administration for managing wet age-related macular degeneration.
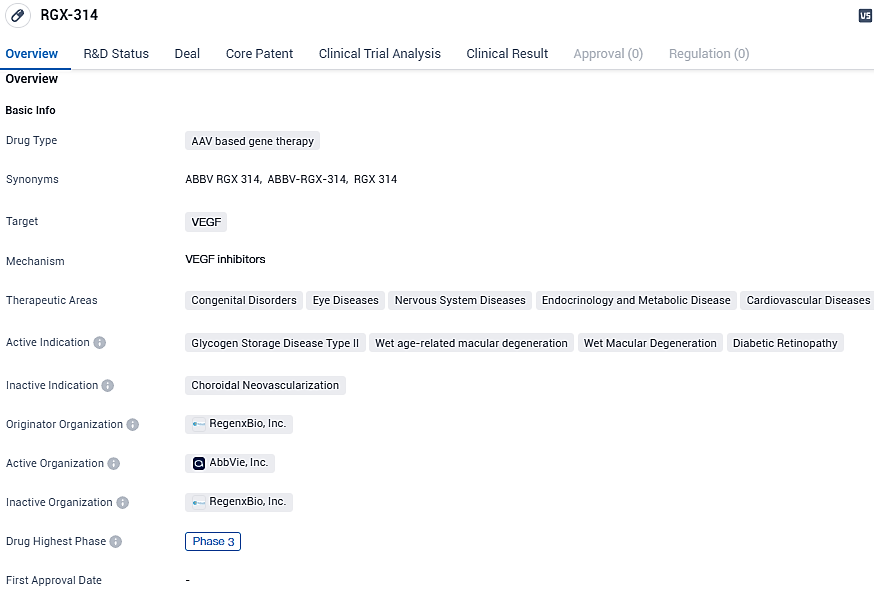
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Wet AMD is an enduring, lifelong condition with existing treatments utilizing anti-VEGF therapies to lower the likelihood of vision loss, although these treatments typically involve repeated injections. An investigatory treatment, ABBV-RGX-314, administered via the suprachoroidal route, aims to provide a single, in-clinic procedure capable of offering enduring anti-VEGF effects, potentially stabilizing or enhancing vision for those living with wet AMD over an extended period.
At the Hawaiian Eye and Retina conference in Maui, HI, new findings were shared by John Pitcher, M.D., from Eye Associates of New Mexico. This included data over six months from two groups receiving the third dose level of ABBV-RGX-314. Co-developed with AbbVie, ABBV-RGX-314 is being examined as a possible singular gene therapy application to address diabetic retinopathy and other persistent retinal diseases.
"We've seen promising evidence that ABBV-RGX-314 could significantly change the approach to managing longstanding retinal diseases, with solid results garnered from research utilizing both subretinal and suprachoroidal administration techniques," conveyed Steve Pakola, M.D., the Chief Medical Officer at REGENXBIO.
Dr. Pakola added, "We're observing good tolerance levels in wet AMD patients receiving a single session treatment with ABBV-RGX-314. It is particularly encouraging to note in this study that the third dose level participants have not developed any inflammation when given a preventative, brief course of topical steroids."
Dr. Pitcher noted, "In real-world settings, we see that the high frequency of injections required for managing wet AMD results in a care regime that many patients find difficult to maintain. Consequently, a number of these patients suffer notable deterioration in vision. Given this, ABBV-RGX-314's strong safety indications and the potential for a long-lasting, single-treatment through suprachoroidal injection could fulfill a critical need for these individuals."
Together with AbbVie, the development of ABBV-RGX-314 is focused on its potential as a one-time therapeutic solution for wet AMD, diabetic retinopathy, and various other ongoing retinal issues. It utilizes the NAV® AAV8 vector that conveys a gene coding for an antibody fragment, purposed to suppress VEGF. This suppression action provided by ABBV-RGX-314 is proposed to obstruct the VEGF pathway, known for its role in the development of new, permeable blood vessels and consequent fluid build-up in the retina.
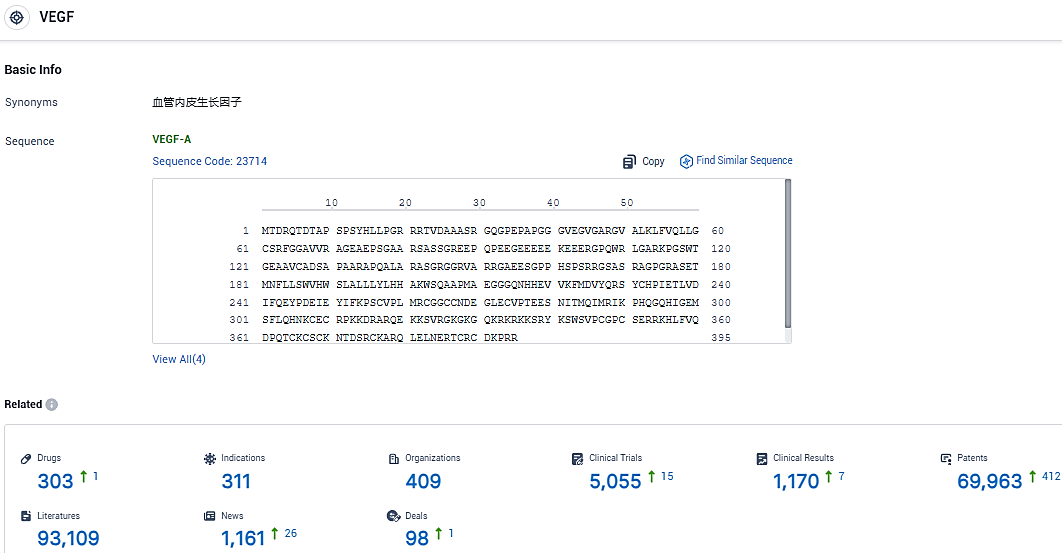
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of January 23, 2024, there are 303 investigational drugs for the VEGF target, including 311 indications, 409 R&D institutions involved, with related clinical trials reaching 5055, and as many as 69963 patents.
REGENXBIO is advancing research in two separate routes of administration of ABBV-RGX-314 to the eye, through a standardized subretinal delivery procedure as well as delivery to the suprachoroidal space. REGENXBIO has licensed certain exclusive rights to the SCS Microinjector® from Clearside Biomedical, Inc. to deliver gene therapy treatments to the suprachoroidal space of the eye.