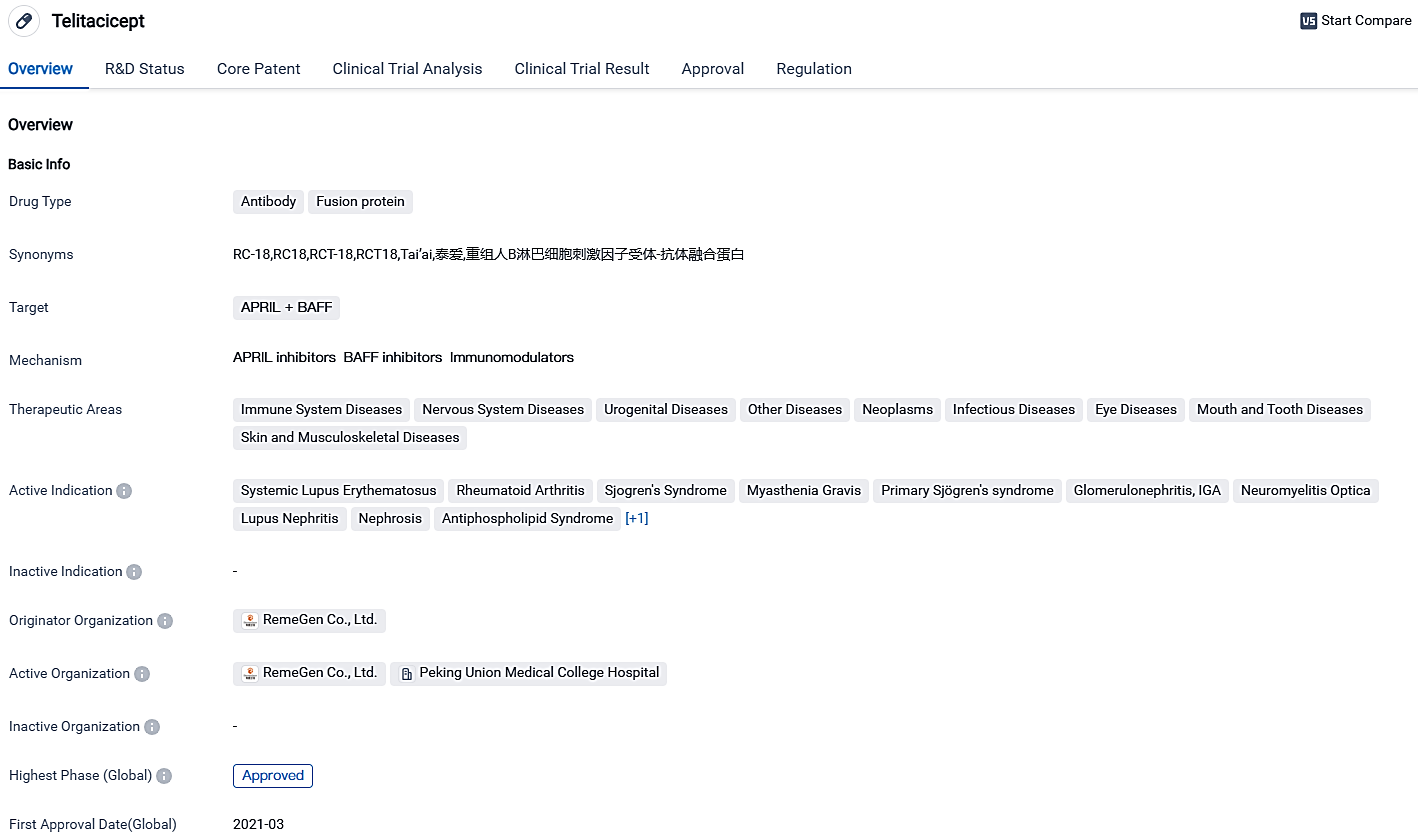
Remegen's new drug Telitacicept (RC18) yields positive Phase III results, applies for additional use in treating rheumatoid arthritis
RemeGen Co., Ltd., a fully-integrated, operational-stage biotech firm has reported recent favorable results from the Phase III clinical trials of Telitacicept(RC18) injection, aimed at treating RA patients in China. Concurrently, a NDA has been sent for review to NMPA in China. Since getting the nod for systemic lupus erythematosus in March 2021, this marks the second time Telitacicept gets an approval for a different indication.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The latest NDA follows a Phase III clinical trial which was randomized, double-blind, placebo-controlled, and spanned across several centers. It saw the participation of 479 rheumatoid arthritis patients. The comprehensive analysis revealed that at week 24, the ACR20 response rate was noticeably greater in patients administered with a dosage of 160mg of Telitacicept and methotrexate compared to those who were only given methotrexate therapy.
Telitacicept is a groundbreaking drug that combines B-cell lymphocyte stimulator/proliferation inducing ligand (BLyS/APRIL) dual-target fusion protein, and is an exclusive development of RemeGen. It curbs abnormal B cell differentiation by repressing the hyperexpression of both cytokines, BLyS and APRIL, hence treating a range of B cell mediated immune diseases.
RemeGen’s CEO and Chief Scientific Officer, Dr. Jianmin Fang, described RA as an enduring autoimmune illness primarily presenting with erosive arthritis, affecting a high global population, regrettably. He anticipates that the latest successful progress with Telitacicept as an alternative therapy for RA will offer new choices to RA patients worldwide.
A report by Frost & Sullivan suggests the global count of rheumatoid arthritis patients is predicted to touch 42.2 million by 2025 and increase to 45 million by 2030. Contemporary standard therapies for rheumatoid arthritis primarily involve anti-inflammatory drugs, glucocorticoids, conventional immunosuppressants, and TNF-α inhibitors.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
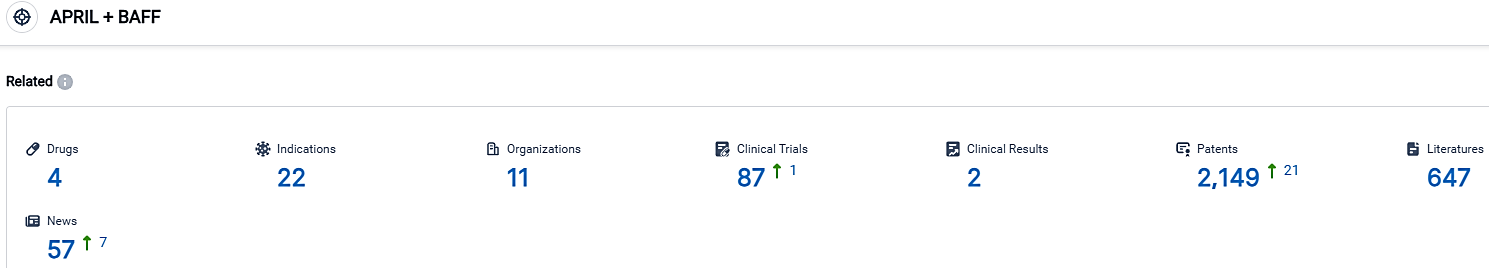
According to the data provided by the Synapse Database, As of October 2, 2023, there are 4 investigational drugs for the APRIL and BAFF target, including 22 indications,11 R&D institutions involved, with related clinical trials reaching 87,and as many as 2149 patents.
Telitacicept(RC18) is a unique fusion protein developed by RemeGen aiming to combat autoimmune diseases. The therapeutic agent has garnered regulatory statuses including priority evaluation and innovative therapy, suggesting its possible prospects as an effective therapy choice.