Decoding Lurbinectedin: A Comprehensive Study of its R&D Trends and Mechanism on Drug Target
Lurbinectedin's R&D Progress
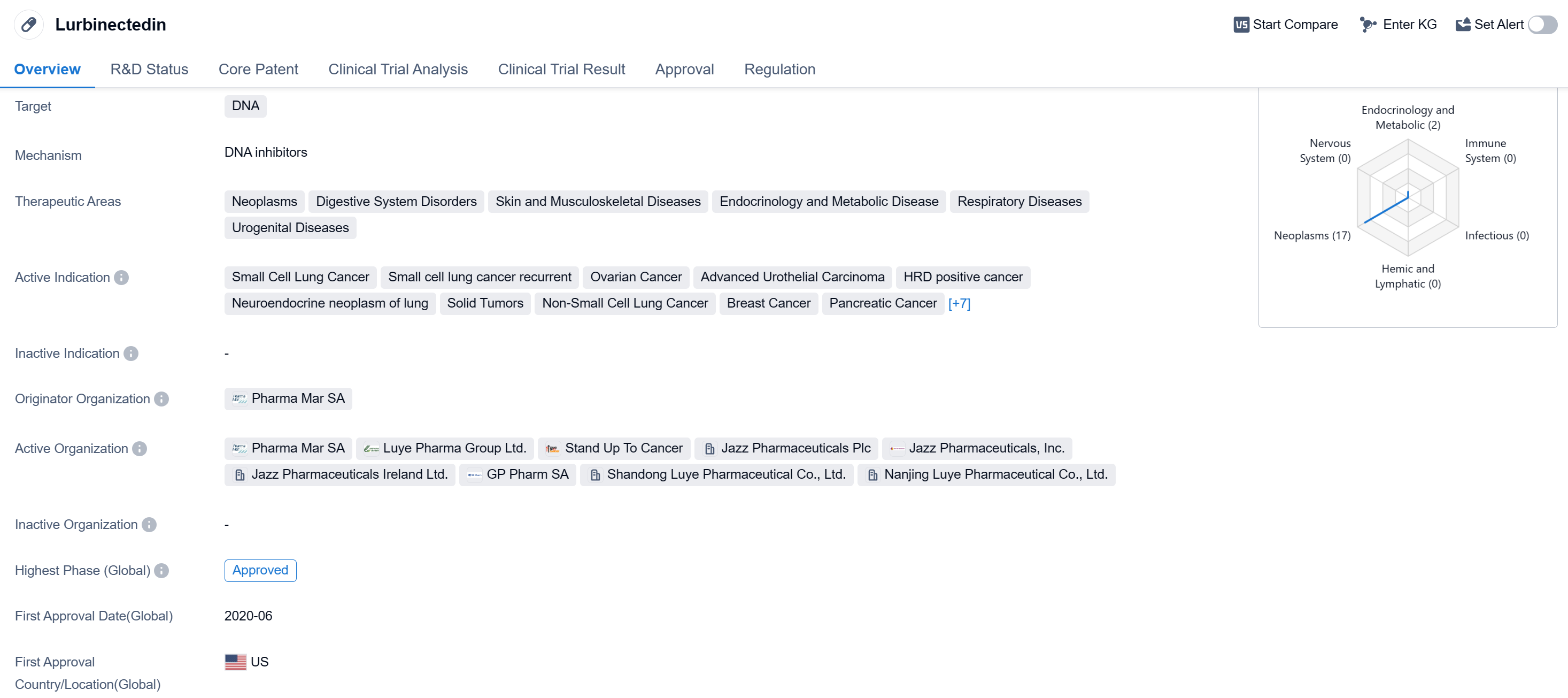
Lurbinectedin is a small molecule drug that targets DNA and has shown potential in treating various therapeutic areas including neoplasms, digestive system disorders, skin and musculoskeletal diseases, endocrinology and metabolic disease, respiratory diseases, and urogenital diseases. It has been indicated for the treatment of small cell lung cancer, small cell lung cancer recurrence, ovarian cancer, advanced urothelial carcinoma, HRD positive cancer, neuroendocrine neoplasm of lung, solid tumors, non-small cell lung cancer, breast cancer, pancreatic cancer, desmoplastic small round cell tumor, embryonal sarcoma of liver, Ewing Sarcoma, recurrent Ewing Sarcoma, refractory Ewing Sarcoma, soft tissue sarcoma, and leukemia.
The drug was developed by Pharma Mar SA and has reached the highest phase of approval globally. It received its first approval in the United States in June 2020. Lurbinectedin has also undergone regulatory processes such as priority review, overseas new drugs urgently needed in clinical settings, accelerated approval, emergency use authorization, and has been designated as an orphan drug.
Lurbinectedin's mechanism of action, targeting DNA, suggests its potential in inhibiting the growth and proliferation of cancer cells. Its approval for various indications indicates its versatility and potential in treating different types of cancers. The drug's small molecule nature may offer advantages in terms of formulation and delivery.
The approval of lurbinectedin in the United States and its highest phase of approval globally highlight its potential as a promising treatment option for patients with the indicated diseases. The drug's designation as an orphan drug suggests its potential in addressing unmet medical needs for rare diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Lurbinectedin: DNA inhibitors
DNA inhibitors are substances or drugs that interfere with the replication or transcription of DNA, thereby inhibiting the growth and division of cells. These inhibitors can target various components of the DNA replication or transcription process, such as enzymes or proteins involved in DNA synthesis or repair.
From a biomedical perspective, DNA inhibitors are of great interest in cancer treatment. Cancer cells often have uncontrolled growth and division due to mutations in their DNA. By targeting and inhibiting specific DNA processes, such as DNA replication or repair, DNA inhibitors can help slow down or stop the growth of cancer cells. This can be achieved by preventing the cancer cells from replicating their DNA or repairing damaged DNA, leading to cell death or reduced tumor growth.
DNA inhibitors can be classified into different types based on their mechanism of action. For example, some DNA inhibitors work by directly binding to DNA and interfering with its structure or function. Others may target enzymes involved in DNA replication or transcription, preventing them from carrying out their normal functions. Examples of DNA inhibitors include chemotherapy drugs like cisplatin, which forms DNA adducts and disrupts DNA replication, and topoisomerase inhibitors, which interfere with the action of enzymes called topoisomerases that are essential for DNA unwinding and replication.
It is important to note that while DNA inhibitors can be effective in treating cancer, they can also have side effects on normal cells that undergo rapid division, such as cells in the bone marrow or gastrointestinal tract. Therefore, the use of DNA inhibitors requires careful consideration and monitoring to balance their therapeutic benefits with potential adverse effects.
Drug Target R&D Trends for Lurbinectedin
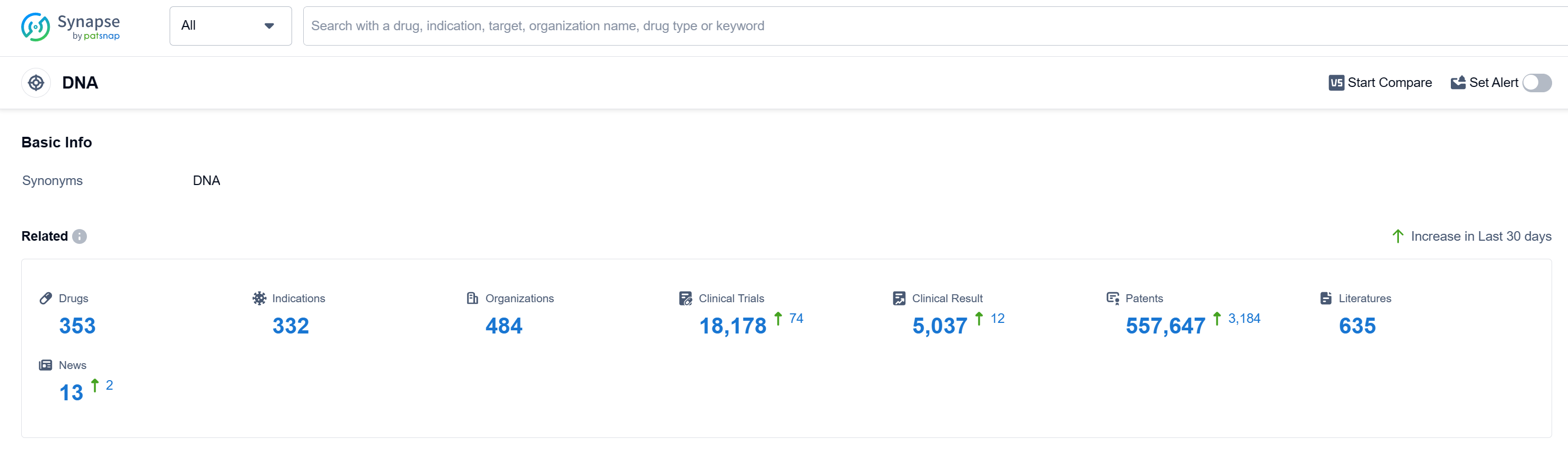
According to Patsnap Synapse, as of 12 Sep 2023, there are a total of 353 DNA drugs worldwide, from 484 organizations, covering 332 indications, and conducting 18178 clinical trials.
The analysis of the current competitive landscape and future development of target DNA in the pharmaceutical industry reveals several key findings. Pfizer Inc., Novartis AG, Baxter International, Inc., Takeda Pharmaceutical Co., Ltd., and Johnson & Johnson are the companies growing fastest under the current target. The most common indications for drugs targeting the DNA include lymphoma, ovarian cancer, breast cancer, neoplasms, and Hodgkin's lymphoma. Small molecule drugs, monoclonal antibodies, and ADCs are the drug types progressing most rapidly under the current target. China, the United States, Japan, and the European Union are the countries/locations developing fastest under the current target DNA, with China showing significant progress. This analysis provides valuable insights into the competitive landscape and future development of target DNA in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Further research and clinical trials may be conducted to explore the full potential of lurbinectedin in treating the indicated therapeutic areas. The drug's approval and regulatory designations provide a solid foundation for its future development and commercialization.