Exploring Moxidectine's Revolutionary R&D Successes and its Mechanism of Action on Drug Target
Moxidectine's R&D Progress
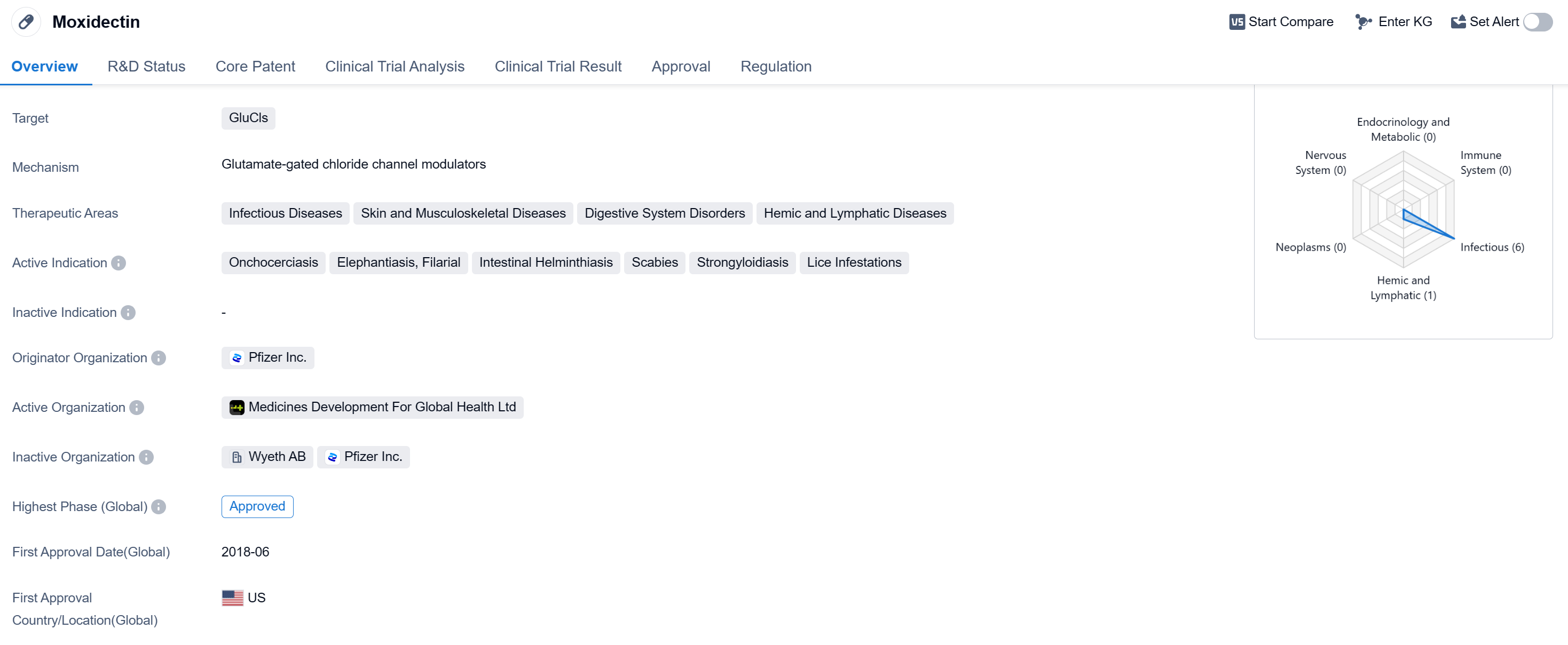
Moxidectin is a small molecule drug that belongs to the class of GluCls-targeting medications. It has been developed primarily for the treatment of various infectious diseases, skin and musculoskeletal diseases, digestive system disorders, and hemic and lymphatic diseases. The drug has shown efficacy in treating conditions such as onchocerciasis, elephantiasis, filarial infections, intestinal helminthiasis, scabies, strongyloidiasis, and lice infestations.
The originator organization of Moxidectin is Pfizer Inc., a renowned pharmaceutical company. The drug has successfully completed its highest phase of development and has received approval for use. The first approval of Moxidectin took place in June 2018 in the United States. It is important to note that Moxidectin has been regulated as an orphan drug.
Moxidectin's approval as an orphan drug signifies its potential in treating rare diseases or conditions that affect a small number of patients. This regulatory status provides certain benefits to the drug's manufacturer, such as market exclusivity and financial incentives, to encourage the development of medications for rare diseases.
The drug's mechanism of action, targeting GluCls, suggests its effectiveness in combating the diseases it is indicated for.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Moxidectine: Glutamate-gated chloride channel modulators
Glutamate-gated chloride channel modulators are substances that can affect the activity of glutamate-gated chloride channels. These channels are ion channels found in the nervous system that are activated by the neurotransmitter glutamate. When these channels are activated, chloride ions flow into the cell, leading to inhibitory effects on neuronal activity.
Glutamate-gated chloride channel modulators can either enhance or inhibit the activity of these channels. By modulating the function of these channels, these substances can have various effects on neuronal excitability and neurotransmission. They can potentially be used as therapeutic agents in the treatment of neurological disorders, such as epilepsy, by either enhancing inhibitory effects or reducing excitatory effects in the brain.
From a biomedical perspective, understanding the modulation of glutamate-gated chloride channels is important for studying the mechanisms of neuronal communication and developing new treatments for neurological conditions.
Drug Target R&D Trends for Moxidectine
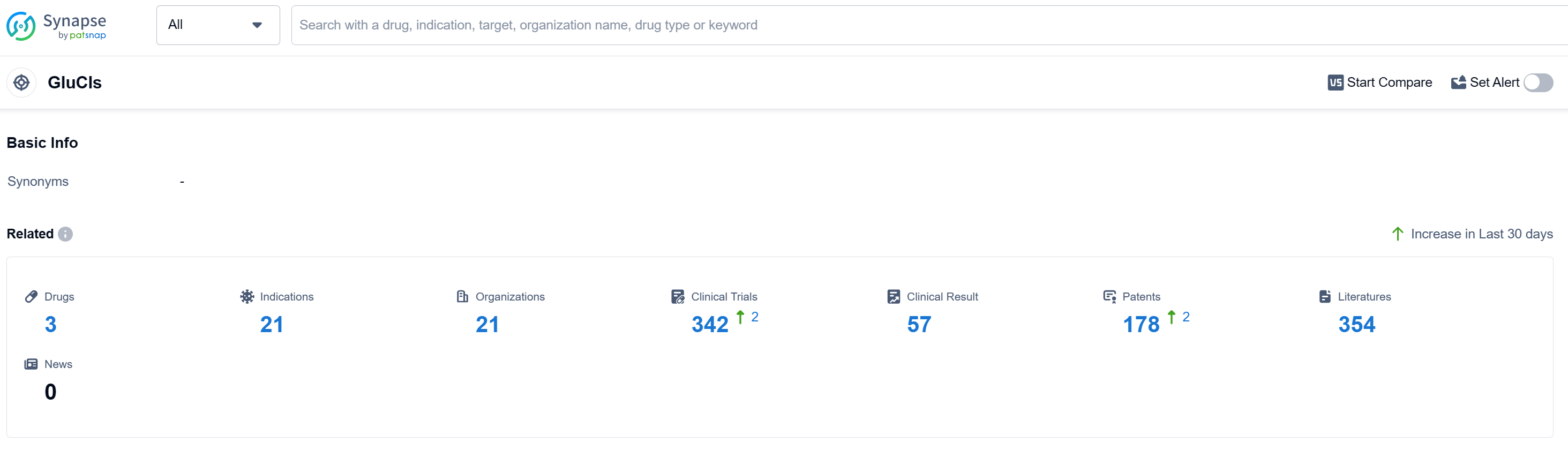
According to Patsnap Synapse, as of 12 Sep 2023, there are a total of 3 GluCls drugs worldwide, from 21 organizations, covering 21 indications, and conducting 342 clinical trials. The analysis of target GluCls in the pharmaceutical industry reveals a competitive landscape with several companies actively involved in research and development. Tarsus Pharmaceuticals, Inc. and Medicines Development For Global Health Ltd have made significant progress, with drugs approved for target GluCls. The approved drugs target a wide range of indications, demonstrating the effectiveness of GluCls in treating various diseases and conditions. Small molecule drugs are progressing rapidly under the target GluCls, indicating less competition from biosimilars. The United States and China are the leading countries in terms of development and approval of drugs targeting GluCls. Overall, the future development of target GluCls shows promising potential for advancements in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Moxidectin is a small molecule drug developed by Pfizer Inc. It has been approved for use in the treatment of various infectious diseases, skin and musculoskeletal diseases, digestive system disorders, and hemic and lymphatic diseases. The drug targets GluCls and has shown efficacy in treating conditions such as onchocerciasis, elephantiasis, filarial infections, intestinal helminthiasis, scabies, strongyloidiasis, and lice infestations. Moxidectin received its first approval in the United States in June 2018 and is regulated as an orphan drug, indicating its potential in addressing rare diseases.