Deep Scientific Insights on Lidocaine hydrochloride's R&D Progress, Mechanism of Action, and Drug Target
Lidocaine hydrochloride's R&D Progress
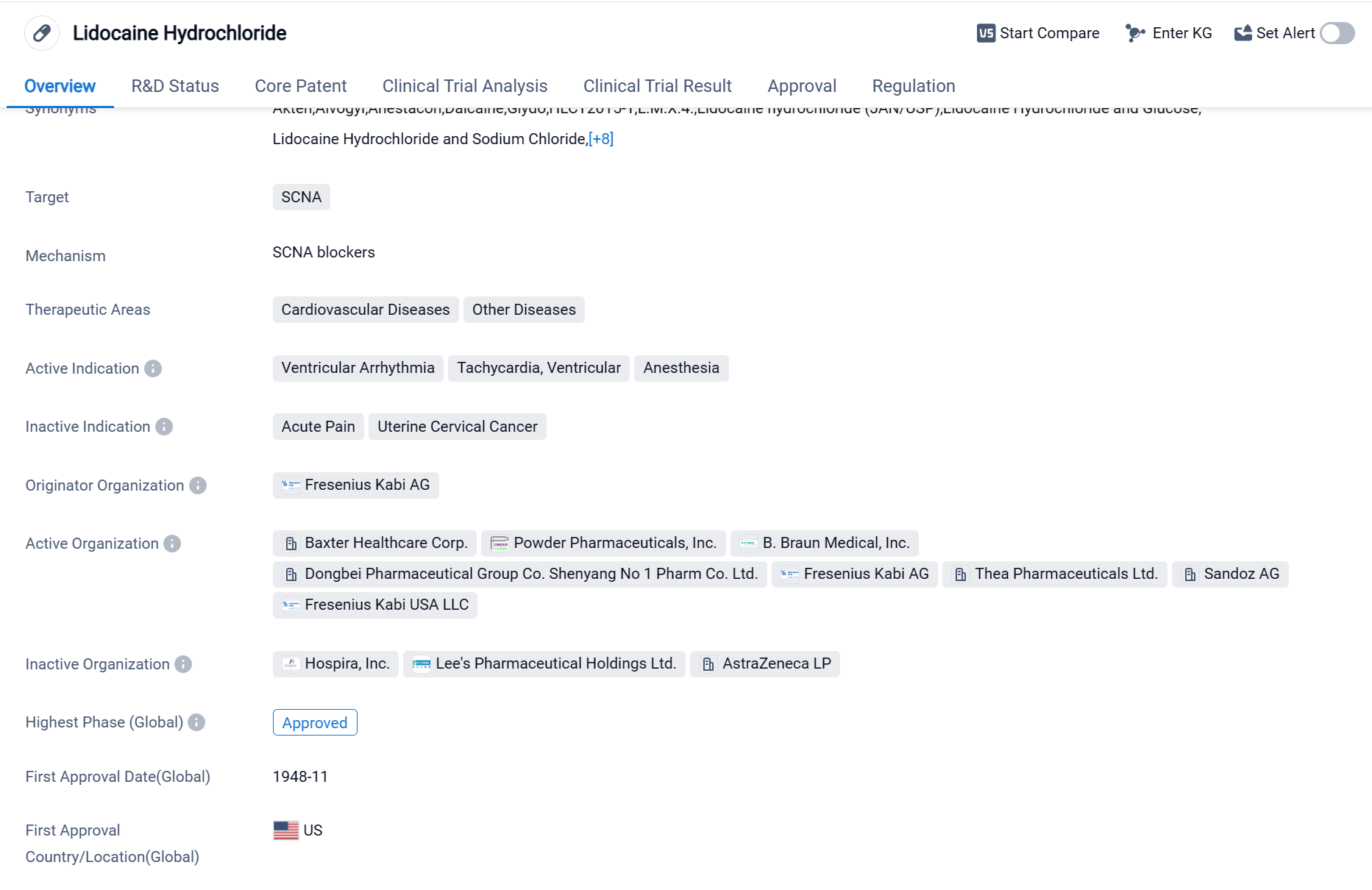
Lidocaine Hydrochloride is a small molecule drug that targets the SCNA protein. It is primarily used in the treatment of cardiovascular diseases and other diseases. The drug has been approved for the treatment of ventricular arrhythmia, tachycardia, ventricular, and anesthesia.
The originator organization of Lidocaine Hydrochloride is Fresenius Kabi AG. The drug has reached the highest phase of approva globally. It was first approved for use in the United States in November 1948. The approval process for Lidocaine Hydrochloride was given priority review, indicating its potential significance in addressing medical needs.
Lidocaine Hydrochloride is widely used in the medical field due to its effectiveness in managing various cardiovascular conditions. Ventricular arrhythmia refers to abnormal heart rhythms originating from the ventricles, the lower chambers of the heart. Tachycardia, ventricular, is a condition characterized by a rapid heartbeat originating from the ventricles. These conditions can be life-threatening and require immediate medical attention. Lidocaine Hydrochloride is administered to patients to restore normal heart rhythm and prevent further complications.
Additionally, Lidocaine Hydrochloride is used as a local anesthetic during surgical procedures or medical interventions. It works by blocking nerve signals in a specific area, numbing the region and reducing pain sensations. This makes it a valuable tool for medical professionals in performing procedures that may otherwise cause discomfort or pain to patients.
The approval of Lidocaine Hydrochloride in both the United States and China highlights its global recognition and acceptance as a safe and effective medication. Its long history of use since 1948 demonstrates its established position in the pharmaceutical industry. The priority review given to the drug suggests that it addresses a significant medical need and has the potential to make a substantial impact in the field of biomedicine.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Lidocaine hydrochloride: SCNA blockers
SCNA blockers are a type of drug that inhibit the activity of sodium channels in the body. Sodium channels play a crucial role in the generation and propagation of electrical signals in cells, particularly in nerve and muscle cells. By blocking these channels, SCNA blockers can modulate the electrical excitability of cells and have various therapeutic applications.
From a biomedical perspective, SCNA blockers are often used in the treatment of neurological disorders such as epilepsy and neuropathic pain. By reducing the excessive electrical activity in the brain or nerves, these blockers help to alleviate seizures and pain symptoms. Additionally, SCNA blockers can also be used as local anesthetics to numb specific areas of the body during surgical procedures.
It's important to note that SCNA blockers can have side effects, as they can affect the normal functioning of sodium channels in other tissues. Common side effects may include dizziness, drowsiness, and gastrointestinal disturbances. Therefore, the use of SCNA blockers should be carefully monitored and administered under medical supervision.
Drug Target R&D Trends for Lidocaine hydrochloride
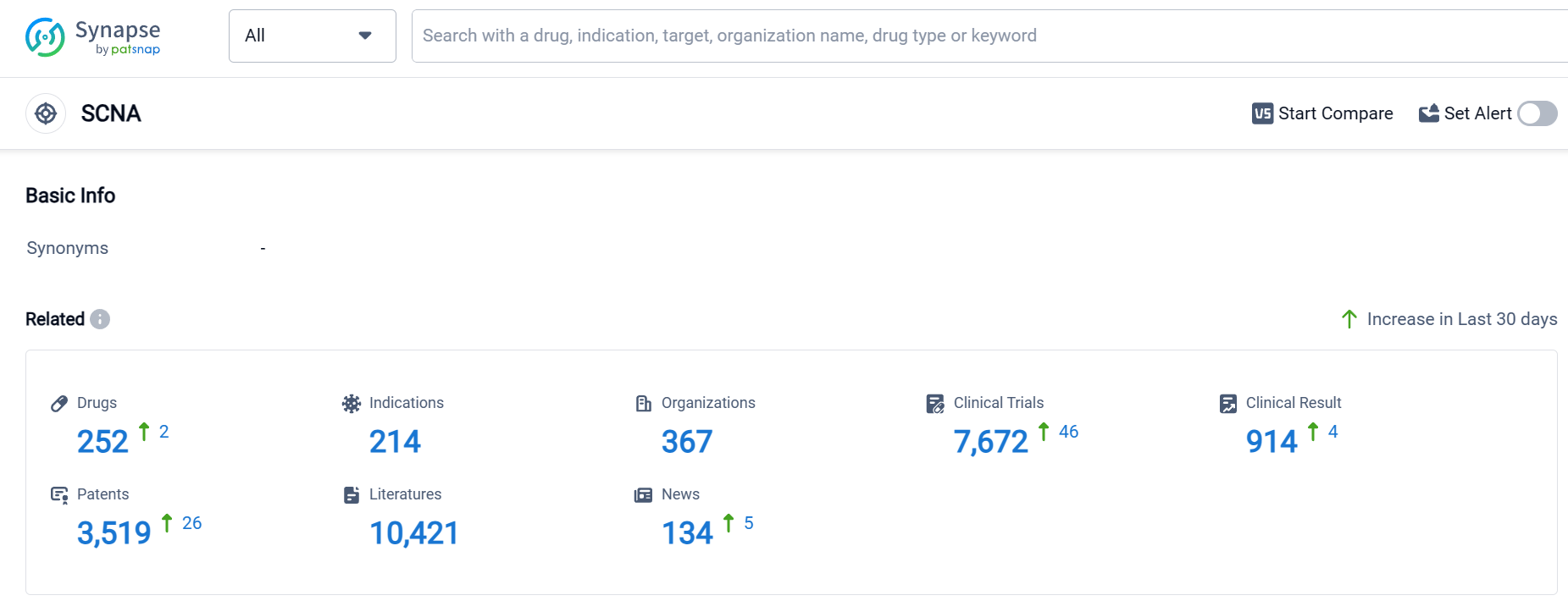
According to Patsnap Synapse, as of 17 Sep 2023, there are a total of 252 SCNA drugs worldwide, from 367 organizations, covering 214 indications, and conducting 7672 clinical trials.
The analysis of the target SCNA from various perspectives provides valuable insights into the current competitive landscape and future development. Novartis AG and Pfizer Inc. are the companies growing fastest under the target SCNA, with a strong focus on R&D. The most approved indications include anesthesia, arrhythmias, cardiac, and pain, indicating a significant need for drugs in these therapeutic areas.
Small molecule drugs dominate the drug type analysis, suggesting intense competition around innovative drugs. China, the United States, and Japan are the countries/locations developing fastest under the target SCNA, with substantial progress in drug development.
Overall, the target SCNA presents a competitive landscape with diverse companies, indications, drug types, and global development efforts. This analysis provides a foundation for further research and strategic decision-making in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Lidocaine Hydrochloride is a small molecule drug developed by Fresenius Kabi AG. It is approved for the treatment of ventricular arrhythmia, tachycardia, ventricular, and anesthesia. The drug targets the SCNA protein and has been widely used since its first approval in 1948. Its priority review status indicates its importance in addressing medical needs, and its global approval signifies its recognition as a valuable medication in the pharmaceutical industry.