Research Progress on GPCR Antibody Drugs
Compared to small molecule drugs, monoclonal antibodies (mAbs) offer several advantages:
mAbs provide better specificity, dosing frequency, and limited central nervous system (CNS) penetration. This makes them potentially effective at interacting with challenging in vivo targets that are difficult for small molecule drugs to achieve.
mAbs exhibit less interpatient variability in pharmacokinetics and can reduce the risk of immunogenicity through the use of human sequences to remove potential T cell epitopes and by selecting appropriate routes of administration.
mAbs not only possess the desired selectivity, good affinity, and longer serum half-life, but also exhibit additional functional effects. These effects are specifically mediated by the Fc region and involve cell killing through antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and antibody-dependent cellular phagocytosis (ADCP).
Moreover, mAbs can be configured in various formats such as antibody fragments, bispecific and multispecific forms, and antibody-drug conjugates (ADCs).
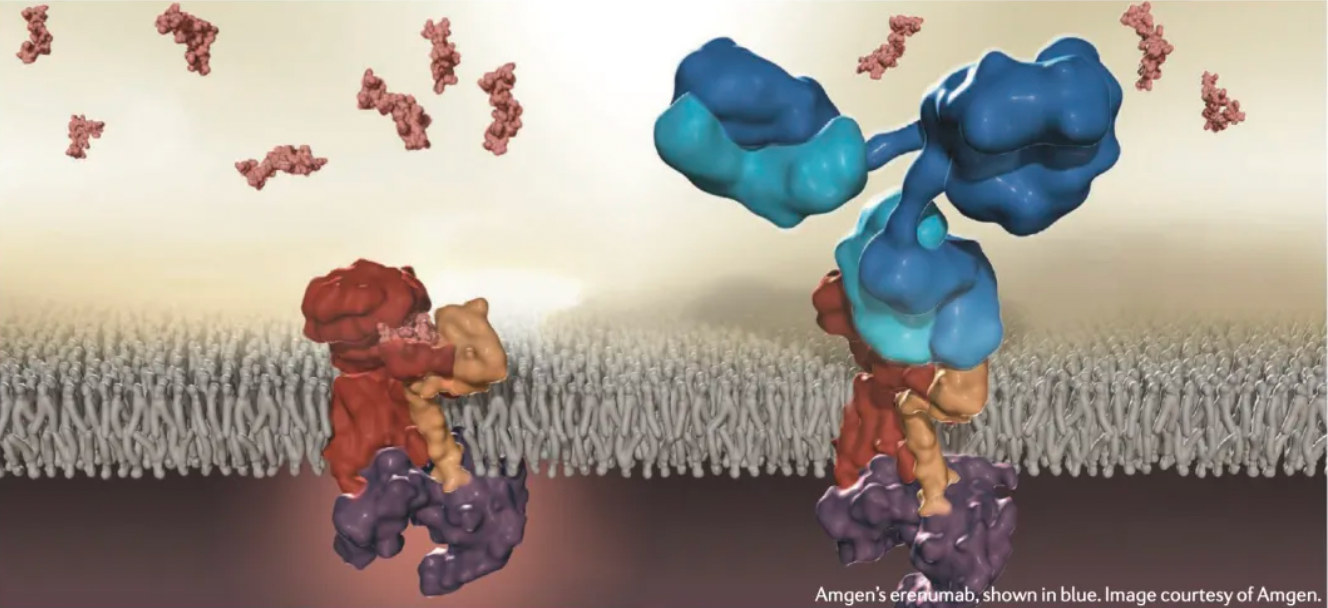
FDA's First GPCR Antibody Drug
Aimovig™ (erenumab) is an injectable human monoclonal antibody (mAb) co-developed and co-marketed by Novartis and Amgen, indicated for the preventive treatment of migraines in adults. Erenumab acts as a selective calcitonin gene-related peptide (CGRP) receptor antagonist and is administered via subcutaneous injection.
Migraine is one of the top ten disabling diseases globally, affecting more than 10% of the population worldwide. Migraines are more complex than simple headaches; they involve debilitating head pain and physical impairment, often accompanied by nausea, vomiting, and tinnitus associated with sound or other sensory disturbances. Migraines also have a significant impact on patients' daily lives, including work productivity and social interactions.
Several potential targets for migraine treatment are currently under investigation. These include adenosine receptors (A1/A2A), pituitary adenylate cyclase-activating polypeptide receptors (PACAP), δ-opioid receptors (DOR), and calcitonin gene-related peptide (CGRP) receptors, all of which are G protein-coupled receptors (GPCRs). Other targets include acid-sensing ion channels (ASIC), transient receptor potential channels (TRP), and potassium channels (KATP), which are ion channels.
The Development History of Aimovig™ (Erenumab)
In 2009, Amgen's team submitted a patent entitled "Human CGRP Receptor Binding Protein," which provided nucleic acids, vectors, and cells encoding antigen-binding proteins. These antigen-binding proteins can inhibit the binding of the CGRP receptor to CGRP, holding potential applications for diseases associated with CGRP receptors, including the treatment and prevention of migraines.
In 2012, Amgen officially initiated a Phase 1 clinical trial for AMG 334 (later known as Aimovig™ (erenumab)), assessing the safety, tolerability, pharmacokinetics, and pharmacodynamics in healthy volunteers and migraine sufferers. This randomized, double-blind, placebo-controlled study included single-dose and ascending multiple-dose arms.
Subsequent data from two Phase 1 clinical trials published in 2017 in Clinical Pharmacology and Therapeutics indicated that AMG 334 exhibited non-linear pharmacokinetics from 1 mg to 70 mg, with a linear clearance phase from 70 mg to 210 mg similar to other human immunoglobulin G2 antibodies. A single dose of AMG 334 antibody showed >75% inhibition of capsaicin-induced skin blood flow with no significant dose dependency observed at doses of 21 mg or higher. Overall, AMG 334 was well-tolerated with an acceptable safety profile, supporting further clinical development for migraine prevention.
Given the promising Phase 1 clinical trial results, in 2013, Amgen initiated multiple Phase 2 clinical studies, starting with a 12-week Phase 2 clinical trial. From August 6, 2013, to June 30, 2014, 483 patients were randomized to receive placebo (n=160), AMG 334 at 7 mg (n=108), 21 mg (n=108), or 70 mg (n=107). At week 12, the mean change in monthly migraine days was -3.4 days for the 70 mg AMG 334 group compared to -2.3 days for the placebo group. The reductions in monthly migraine days for the 7 mg (-2.2 days) and 21 mg (-2.4 days) AMG 334 groups were not significantly different from the placebo. These results suggested that the 70 mg dose of AMG 334 could be a potential therapy for preventing episodic migraines, warranting further investigation in larger Phase 3 trials.
Subsequently, participants from the 12-week double-blind study entered an open-label extension study, receiving 70 mg of AMG 334 every 4 weeks over a longer period. Among the 383 participants, the median treatment duration with AMG 334 was 575 days (range 28-822 days). By week 64, 65%, 42%, and 26% of participants achieved ≥50%, ≥75%, and 100% reduction in monthly migraine days, respectively. The mean HIT-6 score decreased from 60.2 (6.3) at baseline to 51.7 (9.2) at week 64. Improvements in MSQ and MIDAS scores were sustained through week 64.
The long-term efficacy and safety of AMG 334 for migraine prevention were demonstrated in the results of this 5-year open-label extension phase of a randomized clinical trial.
The world's first GPCR monoclonal antibody drug
Mogamulizumab (Poteligeo), a humanized, afucosylated IgG1 monoclonal antibody specifically targeting CCR4, was developed by Kyowa Hakko Kirin in Japan. By binding to the N-terminal domain of CCR4, mogamulizumab induces antibody-dependent cellular cytotoxicity.
Mogamulizumab was first approved in Japan in 2012 for the treatment of relapsed or refractory CCR4+ adult T-cell leukemia/lymphoma (ATCL) and was later approved in 2014 for relapsed or refractory CCR4+ cutaneous T-cell lymphoma (CTCL).
In August and November 2018, the FDA and EMA respectively approved mogamulizumab for the treatment of adult patients with relapsed or refractory mycosis fungoides (MF) or Sézary syndrome (SS) who have received at least one prior systemic therapy.
According to results from a phase 3 clinical study published in The Lancet Oncology in 2018, mogamulizumab significantly extended progression-free survival compared to the small molecule drug vorinostat, offering a new effective treatment option for patients with cutaneous T-cell lymphoma.
The World's First GPCR Bispecific Antibody Drug
Talquetamab is a bispecific T-cell engaging antibody that targets both the CD3 receptor on T cells and GPRC5D. GPRC5D, a novel drug target, is a Class C G protein-coupled receptor that is present on some normal cells but overexpressed on myeloma cells.
According to information about Talquetamab, this antibody is a fully humanized IgG4 bispecific antibody developed using hybridoma and phage display platforms. It has undergone humanization engineering and is administered subcutaneously. In 2017, the Johnson & Johnson team first submitted a global patent (WO2018017786) for the development of Talquetamab, mentioning that this patent provides an antibody specific to GPRC5D. The provided antibody can be used to diagnose, treat, or monitor the progression, remission, or stabilization of cancers expressing GPRC5D; to determine whether a patient should receive cancer treatment; or to ascertain whether a subject has a cancer expressing GPRC5D and thus may be eligible for GPRC5D-specific anticancer therapy.
Surrounding the discovery of GPRC5D x CD3-related antibodies, the Johnson & Johnson team has obtained approval for a total of 8 related patent applications and has conducted multiple clinical studies focusing on multiple myeloma (MM) and relapsed/refractory multiple myeloma (RRMM).
In December 2022, the Johnson & Johnson team published an article in the New England Journal of Medicine reporting the results of a Phase 1 clinical trial of the T-cell redirecting GPRC5D bispecific antibody Talquetamab for the treatment of multiple myeloma. The results showed that 232 patients were treated with Talquetamab (102 intravenously, 130 subcutaneously). The median follow-up time was 11.7 months for patients receiving a dose of 405 micrograms and 4.2 months for those receiving 800 micrograms. The response rates were 70% and 64%, respectively. The median duration of response was 10.2 months and 7.8 months, respectively.
For the two subcutaneous injection doses (405 micrograms per kilogram weekly and 800 micrograms per kilogram biweekly), common adverse reactions included cytokine release syndrome (in 77% and 80% of patients, respectively), skin-related events (in 67% and 70%), and oral ulcerations (in 63% and 57%); all except one case of cytokine release syndrome were Grade 1 or 2.
According to the latest information on the Johnson & Johnson website, the FDA submission includes the most recent clinical research data. The Phase 2 MonumenTAL-1 study of Talquetamab included patients (187 individuals) who had received at least four prior therapies and had not previously received T-cell redirecting therapy. The results demonstrated a significant overall response rate (ORR). In the 0.8 mg/kg SC biweekly regimen, 73.6% of patients achieved ORR. With a median follow-up of nearly 6 months (range: 0 to 9.5 months) from the initial response, 58% of patients achieved a very good partial response (VGPR) or better, with 33% achieving complete response (CR) or better.
For the weekly SC dose of 0.4 mg/kg, 73.0% of patients achieved ORR. With a median follow-up of nearly 14 months (range: 0.8 to 15.4) from the initial response, 57% of patients achieved VGPR or better, with 35% achieving CR or better.
Representative ADC Products
Antibody-drug conjugates (ADCs), as an antibody-based therapeutic approach, are among the fastest-growing categories of drugs in cancer treatment. ADCs are essentially monoclonal antibodies (mAbs) that are chemically linked to cytotoxic payloads. These conjugates can specifically recognize and bind to antigens that are expressed at much higher levels on cancer cells compared to normal cells, thereby delivering the drug selectively to the tumor site. Consequently, ADCs improve the therapeutic index by restricting the delivery of potent cytotoxic drugs only to the targeted tumor sites and minimizing nonspecific effects on normal tissues.
Currently, 12 ADCs have been approved by the U.S. Food and Drug Administration (FDA), and dozens more are at different stages of preclinical and clinical development. Notably, 8 ADCs have been approved in the past five years alone, underscoring the rapid progress in this field. ADCs have been approved for treating both hematologic malignancies and solid tumors, with indications including acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), multiple myeloma (MM), HER2-positive breast cancer, triple-negative breast cancer, gastric cancer, and several other diseases.
Since the first ADC was introduced to the market in 2000, ADC technology has undergone extensive improvements to enhance efficacy and safety. As ADC research and development continue, the range of target antigens is also expanding. G-protein-coupled receptors (GPCRs) are emerging as targets for ADCs and have the potential to significantly broaden the current range of ADC target antigens. Multiple GPCRs have already been targeted by ADCs, with two entering Phase I clinical trials.
LaNova Medicines: LM-305
On May 23, LaNova Medicines signed an exclusive licensing agreement with AstraZeneca for LM-305. Under the terms of the agreement, AstraZeneca will obtain exclusive global rights to research, develop, and commercialize LM-305. LaNova Medicines stands to receive up to $55 million in upfront and near-term payments, and up to $545 million in additional development and commercial milestones, as well as tiered royalties on global net sales.
LM-305 is the second antibody-drug conjugate (ADC) developed by LaNova Medicines using its proprietary LX-ADC™ platform. It is the first GPRC5D-targeting macromolecular new drug from China and the first GPRC5D-targeting ADC to enter clinical stages worldwide.
GPRC5D is an orphan class C G protein-coupled receptor (GPCR) whose function in human tissues remains unclear. Research indicates that GPRC5D is primarily expressed in malignant bone marrow plasma cells and hair follicles, with minimal or no expression in normal tissues. Because GPRC5D is highly overexpressed in patients with multiple myeloma and its expression is relatively independent of another multiple myeloma target, BCMA, GPRC5D is considered a promising target for treating relapsed/refractory multiple myeloma (RRMM).
According to LaNova Medicines' published patents, the discovery of GPRC5D-targeting antibodies involved immunizing mice with GPRC5D protein and screening through hybridomas, resulting in over 20 hybridomas that underwent subsequent antibody characterization.
LaNova's team further proceeded with antibody labeling and internalization efficiency detection using pHAb dyes, ADCC activity analysis using reporter gene assays, antibody humanization modifications, and activity evaluations of the humanized antibodies. These studies demonstrated that the optimized humanized antibodies exhibited strong internalization activity in both GPRC5D overexpressing cell lines and endogenous multiple myeloma cell lines.
Subsequently, LaNova Medicines developed two ADC products, antibodies 37B9C4 and 58F9G10, each conjugated with the cytotoxic agent MMAE, to study their cytotoxic activity against target cells and antitumor efficacy. Among them, LM-305 exhibited very promising antitumor effects.
Takeda: TAK-500
TAK-500 is an ADC (Antibody-Drug Conjugate) product developed by the Takeda team. It specifically targets STING and CCR2 and can selectively activate STING in cells expressing CCR2. This approach facilitates the accumulation of STING agonists within tumors, potentially exerting antitumor activity.
The stimulator of interferon genes (STING) is an endoplasmic reticulum membrane protein encoded by the STING1 gene, playing a critical role in innate immunity. When cells are infected by intracellular pathogens, such as viruses, fungi, or intracellular parasites, STING induces the production of type I interferons. Type I interferons protect infected cells and neighboring cells from localized infections through autocrine or paracrine signaling.
The chemokine receptor CCR2 is highly expressed in tumor-infiltrating myeloid cells, promoting immune evasion by restricting CD8+ T-cell infiltration. Myeloid cells are present in the tumor microenvironment of most human solid tumors and play a crucial role in influencing local immunoregulatory functions.
Activating STING signaling in intratumoral myeloid cells enhances interferon production, thereby boosting local innate and adaptive immunity and synergizing with other antitumor mechanisms. By targeting STING to myeloid cells expressing CCR2, TAK-500 may operate through three potential mechanisms: activating the IFN response, reprogramming inhibitory intratumoral CCR2+ cells to an inflammatory phenotype, and blocking the recruitment of inhibitory tumor-associated macrophages (TAMs).
TAK-500 is composed of three parts: an anti-CCR2 IgG1 antibody, a STING agonist TAK-676 developed by Takeda, and a protease-cleavable maleimide linker.
TAK-676 is a STING agonist developed by the Takeda team, capable of triggering the STING signaling pathway and activating type I interferons. TAK-676 also acts as an immune system modulator, potentially restoring the immune system and generating durable memory T-cell immunity. TAK-676 is currently undergoing multiple clinical studies for its antitumor effects.
According to a China patent (CN 116490244 A) disclosed by Takeda in 2022 regarding STING agonists, the STING agonist TAK-676 (Compound 14) or its pharmaceutically acceptable salts, combined with one or more immune checkpoint inhibitors and radiation, is being developed for cancer therapy.
In April 2023, the Takeda team presented a poster at AACR detailing the clinical-stage TAK-500, which can induce STING activation in intratumoral myeloid cells expressing CCR2, producing favorable immunomodulatory effects.
Preclinical results indicate that treatment with mTAK-500 in synthetic mouse models with intratumoral myeloid cells expressing CCR2 leads to the accumulation and activation of CD8+ effector T cells in the tumor microenvironment, resulting in significant antitumor activity and improved survival rates. The baseline levels of mMDSCs expressing CCR2 in the mouse models positively correlate with the antitumor response of mTAK-500.
An ongoing open-label Phase 1a/1b clinical study (NCT05070247) posted on clinicaltrials.gov is evaluating the safety, tolerability, antitumor activity, pharmacokinetics, and pharmacodynamics of TAK-500 in patients aged ≥18 years with gastroesophageal adenocarcinoma, pancreatic adenocarcinoma, hepatocellular carcinoma, non-squamous non-small cell lung cancer, head and neck squamous cell carcinoma, mesothelioma, or triple-negative breast cancer. Eligibility criteria include disease progression or intolerance to all standard therapies.
Daiichi Sankyo: DS-6157a
GPR20 is an orphan receptor belonging to the GPCR family class A. Based on research evidence indicating its selective high expression in GIST (Gastrointestinal Stromal Tumor), the Daiichi Sankyo team developed an anti-GPR20 ADC (Antibody-Drug Conjugate) drug, DS-6157a, designed to deliver the cytotoxic DNA topoisomerase I inhibitor exatecan directly to the tumor.
In June 2021, the Daiichi Sankyo team published their findings in the journal "Cancer Discovery," reporting that their anti-GPR20 ADC drug DS-6157a exhibited GPR20 expression-dependent antitumor activity in GIST xenograft models, including those resistant to Imatinib, Sunitinib, and Regorafenib.
First, the researchers used DNA immunization to generate three rat monoclonal antibodies (mAbs) targeting human GPR20, named 04-046, 04-093, and 04-021. Epitope validation further demonstrated that 04-046 binds to the extracellular ECD1 and ECD2 domains of the receptor.
The researchers humanized the 04-046 monoclonal antibody to reduce immunogenicity and used this antibody alongside DXd-ADC technology to create the anti-GPR20 ADC candidate drug DS-6157a.
Studies on various GIST tumor models showed that DS-6157a exhibited significant antitumor effects. Additionally, DS-6157a demonstrated superior antitumor efficacy compared to classic tyrosine kinase inhibitors (TKIs), such as the approved drugs Imatinib, Sunitinib, and Regorafenib.
Daiichi Sankyo has extensive experience in developing ADC products, having successfully launched the anti-HER2 product Fam-trastuzumab deruxtecan-NXKI, which was approved by the FDA in 2019. This success earned the product various accolades, including priority review, breakthrough therapy designation, fast track designation, and orphan drug status.
In 2016, the Daiichi Sankyo team first reported in the "Bioorganic and Medicinal Chemistry Letters" journal the use of DXd-ADC technology to develop the anti-HER2 ADC candidate drug Fam-trastuzumab deruxtecan-NXKI for the treatment of certain types of unresectable or metastatic HER2-positive breast cancer.
Based on the preclinical antitumor effects and pharmacokinetic features of DS-6157a, it was deemed a potential new therapy for GIST patients who are refractory, resistant, or intolerant to approved TKIs. Consequently, Daiichi Sankyo registered and initiated a Phase 1 clinical trial for DS-6157a (NCT04276415) in 2020.
In June 2023, the Daiichi Sankyo team published the treatment outcomes of DS-6157a in 34 enrolled patients in the journal "Clinical Cancer Research."
DS-6157a had good tolerability, with dose-limiting toxicity observed in four patients (11.8%) at doses of 6.4 mg/kg, 9.6 mg/kg, and 12.8 mg/kg. The maximum tolerated dose was determined to be 6.4 mg/kg. Seventeen patients (50.0%) experienced ≥Grade 3 treatment-related adverse events, including decreased platelet count (23.5%), anemia (20.6%), neutrophil count decrease (14.7%), and white blood cell count decrease (11.8%). Four patients (11.8%) experienced serious adverse events related to DS-6157a. Six patients died, with five deaths attributed to disease progression and one death to a DS-6157a-related treatment-emergent adverse event (TEAE). Tumor shrinkage was observed in seven patients (20.6%), and one patient (2.9%) achieved a partial response.
Due to the lower-than-expected efficacy, further clinical studies on DS-6157a were discontinued, marking the failure of the first ADC product targeting the orphan receptor GPR20.
Novartis: JBH-492
JBH-492 is an ADC (antibody-drug conjugate) product developed by the Novartis team, specifically targeting CCR7. It comprises a humanized anti-CCR7 IgG1 antibody linked via a cleavable linker to the antimitotic agent DM4, which inhibits microtubule polymerization and cell division.
The development details of this ADC drug are based on the Novartis team's 2018 patent submission titled “Anti-CCR7 Antibody Drug Conjugates.”
Researchers established CCR7-stably expressing cell lines, virus-like particles (VLP), and protein constructs. Utilizing the three extracellular loops of CCR7, they constructed a protein construct FabCCR7M1 with a Fab scaffold that can display the extracellular loops of CCR7 in a soluble form, suitable for animal immunization and antibody generation.
Through repeated immunizations in Bcl-2 transgenic mice, hybridoma production, FACS screening, antibody purification, and antibody humanization, they produced a series of high-affinity anti-CCR7 antibodies.
Multiple humanized anti-CCR7 antibodies exhibited high affinity for CCR7-expressing VLPs, with some showing pH-dependent binding characteristics.
Subsequently, researchers used the PathHunter assay to evaluate the agonistic or antagonistic functions of these antibodies. The results indicated that none of the antibodies exhibited agonistic activity. However, several antibodies, including 506E15 and 121G12, were identified as potent antagonists capable of blocking the binding of endogenous ligands.
Further investigations involved labeling anti-CCR7 antibodies in the form of CysMab with malemide-pHrodo to study internalization activity. The figure below illustrates the internalization capabilities of three antibodies in various cell lines.
Using BLI technology and mutagenesis studies, researchers further identified the epitopes recognized by different antibodies. Ultimately, they selected 121G12 for the generation and characterization of the antibody-drug conjugate, resulting in an ADC with a DAR (drug-to-antibody ratio) of approximately 4.
In vivo antitumor studies demonstrated that this series of ADC products exhibited dose-dependent antitumor effects in various tumor xenograft models.
Genentech: RG-7636
RG-7636 (DEDN6526A) is an ADC product developed by Genentech, consisting of a humanized IgG1 anti-ETB receptor monoclonal antibody MEDN6000A and a potent mitotic inhibitor drug, MMAE.
The Genentech team first reported this ETB receptor-specific ADC product for preclinical melanoma studies in the journal Clinical Cancer Research in 2011.
Initially, researchers utilized mRNA transcription analysis to identify the characteristic overexpression of the ETB receptor in 28 different metastatic melanoma cell lines.
By employing wild-type ETB receptors overexpressed in HEK293 cells or purified ETB receptors as immunogens for BALB/c mice, hybridoma technology was utilized to screen and obtain therapeutic antibodies specifically targeting the extracellular region of the ETB receptor.
Antibody 24C7 attracted researchers' attention because ET-1 preincubation treatment could block its binding with the ETB receptor. Further studies indicated that this antibody and ET-1 have mutually exclusive binding with the ETB receptor, competing for the same epitope.
Before making efforts to obtain more suitable therapeutic antibodies, researchers attempted to directly assess the role of the ETB receptor in melanoma in vivo growth. By inducing conditional knockdown of ETB receptor expression via shRNA, there was not a significant inhibition of tumor growth observed. These results, similar to the disappointing outcomes of second-phase clinical studies using endothelin receptor antagonists directly for melanoma treatment, prompted researchers to abandon further investigation of receptor inhibition methods. Instead, they sought to develop potential ADC products targeting the ETB receptor.
Researchers then focused on another monoclonal antibody called 5E9, as this antibody was capable of carrying labeled fluorescent groups into cells and colocalizing with lysosome marker LAMP1 and early endosome marker EEA1 in staining experiments. One key characteristic of an ADC candidate is the ability to deliver the conjugated drug payload into the cell after binding to the cell surface.
The rapid internalization of 5E9 in melanoma cells prompted researchers to adopt an armed antibody approach, conjugating the potent cytotoxic compound MMAE with 5E9. Initially, 5E9 ADC demonstrated specific cytotoxicity relative to control ADC in various cell lines with different levels of ETB receptor expression, which generally correlated with ETB receptor expression.
Further research data indicated that knocking out ETB receptor expression resulted in the loss of 5E9 ADC cytotoxicity, whereas overexpressing ETB receptor expression enhanced cytotoxicity.
Finally, in in vivo efficacy studies, researchers observed that administering 1, 3, and 6 mg/kg doses of 5E9 ADC or a 6 mg/kg dose of control ADC to animals bearing A2058 and UACC-257 tumors resulted in a sustained decrease in tumor volume with high doses of 5E9 ADC.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!