Revolutionizing Antibody Drug Discovery: The Role of AI in Enhancing Design, Optimization, and Affinity Improvement
The application of Artificial Intelligence (AI) in drug discovery is increasingly becoming a key force in driving the pharmaceutical industry forward. AI technologies, especially machine learning (ML) and deep learning (DL), are playing a growing role in various stages of drug development, including target identification, compound screening, and drug design. These advancements significantly enhance the efficiency of new drug development and provide opportunities to reduce costs and improve success rates. For example, the application of AI in drug discovery can reduce the development timeline and increase the success rate of new drugs from 12% to 14%, potentially saving about $55 billion annually in compound screening and clinical trial costs. AI's influence in drug development is crucial for meeting clinical needs and gaining a competitive market edge.
Traditional Antibody Drug Design Approaches:
Traditional methods for antibody design and discovery have historically relied on biotechnological techniques in the laboratory. These methods were the mainstream process for antibody development before the emergence of AI. For instance, hybridoma technology is a classic antibody production method that involves fusing the B-cells of immunized animals with myeloma cells, resulting in hybridoma cells capable of proliferating indefinitely and secreting specific monoclonal antibodies. Additionally, phage display technology uses bacteriophages to present proteins or peptides on their surface. By selecting phages that bind to a target antigen, researchers can identify and select antibody candidates with high affinity.These conventional methods laid a strong foundation for the development of antibody drugs.
Traditional antibody design also includes other techniques such as stimulating an animal's immune system to produce antibodies, antibody humanization to reduce immunogenicity, and affinity maturation to enhance antibody binding affinity. These methods involve complex biochemical and molecular biological operations, requiring extensive laboratory work and significant time investment. However, they provide precise and reliable means for developing antibody drugs.
Application of AI in Antibody Drug Design:
With the development of AI, these traditional methods are now being combined with AI technologies to improve the efficiency and accuracy of antibody discovery, reduce the number of experiments required, shorten development cycles, lower costs, and increase antibody affinity and specificity. AI, particularly machine learning (ML) and deep learning (DL), is playing an increasingly important role in key stages of drug discovery such as target identification, compound screening, and drug design, offering substantial improvements in the overall efficiency of new drug development while lowering costs and increasing the likelihood of success.
AI-Assisted Antibody Structure Design
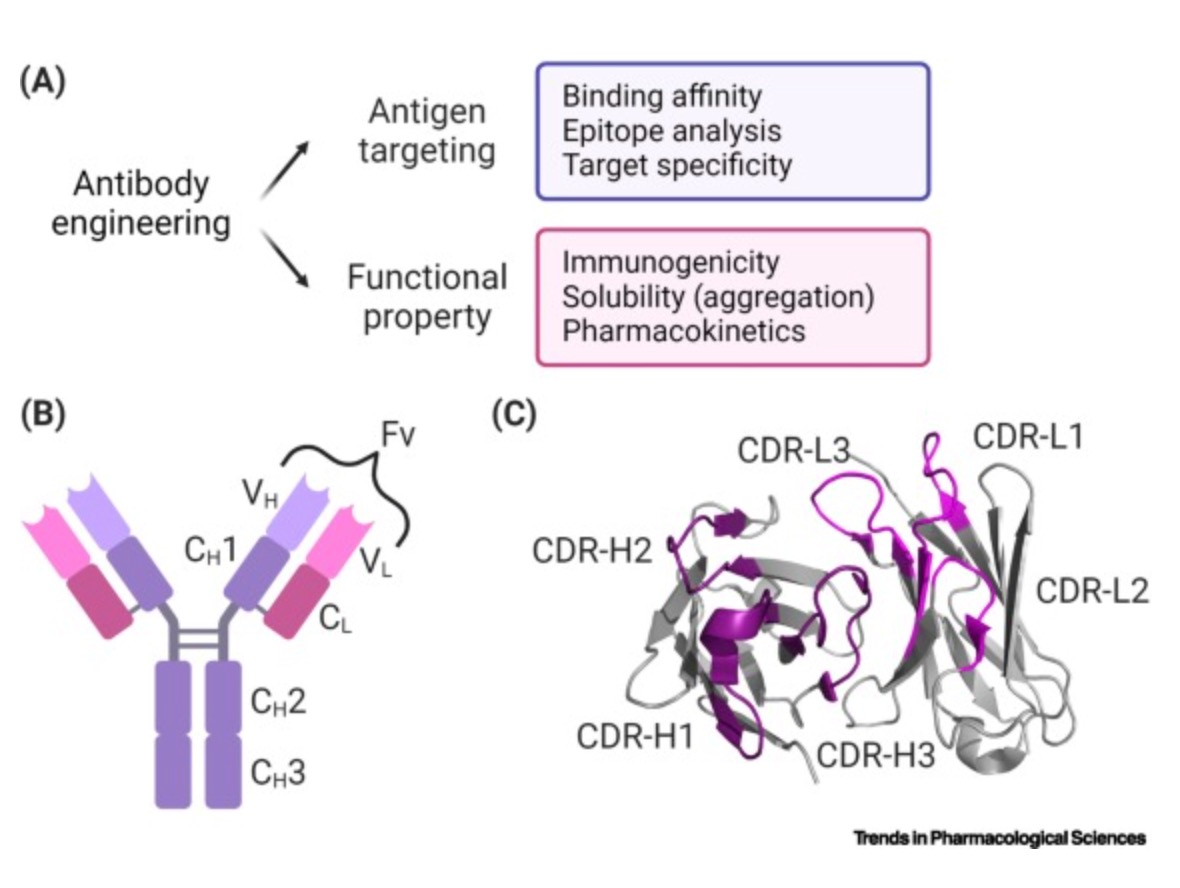
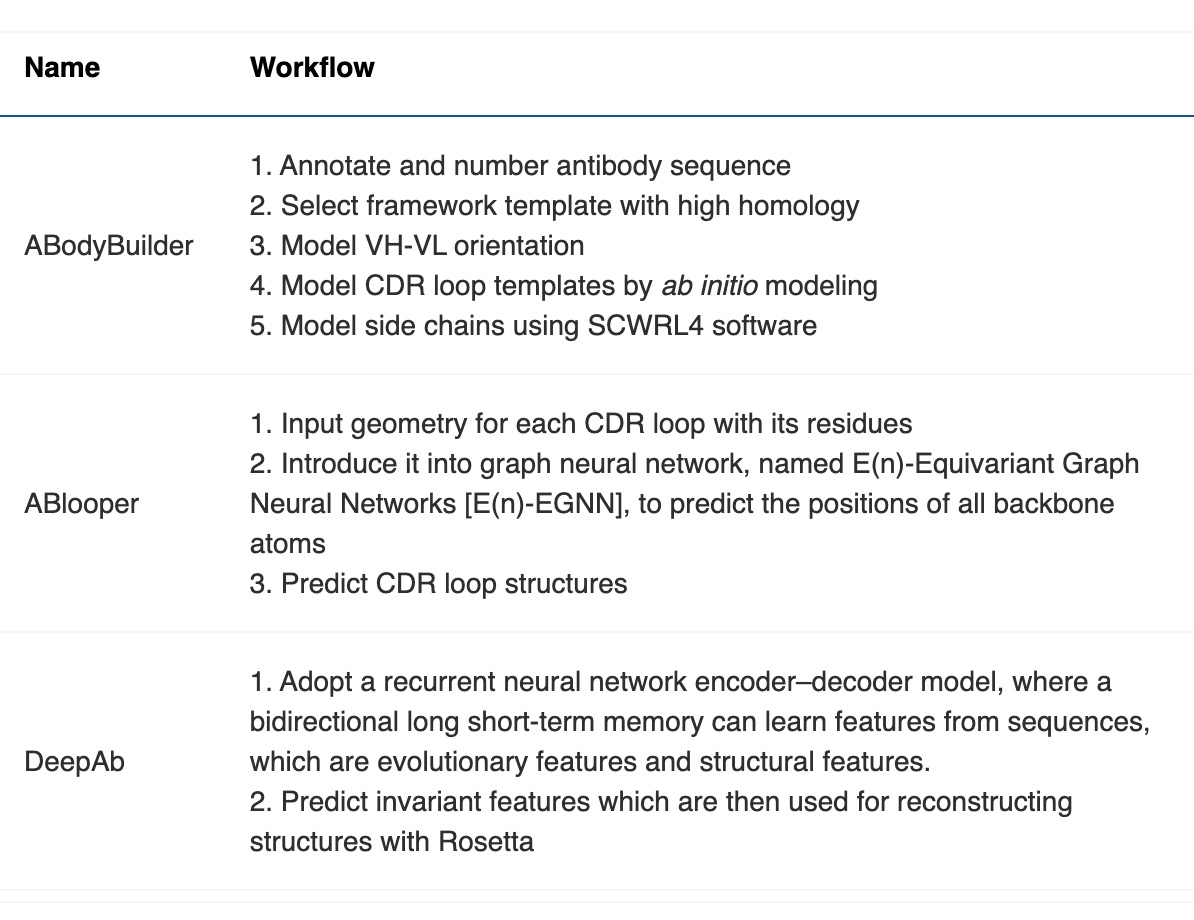
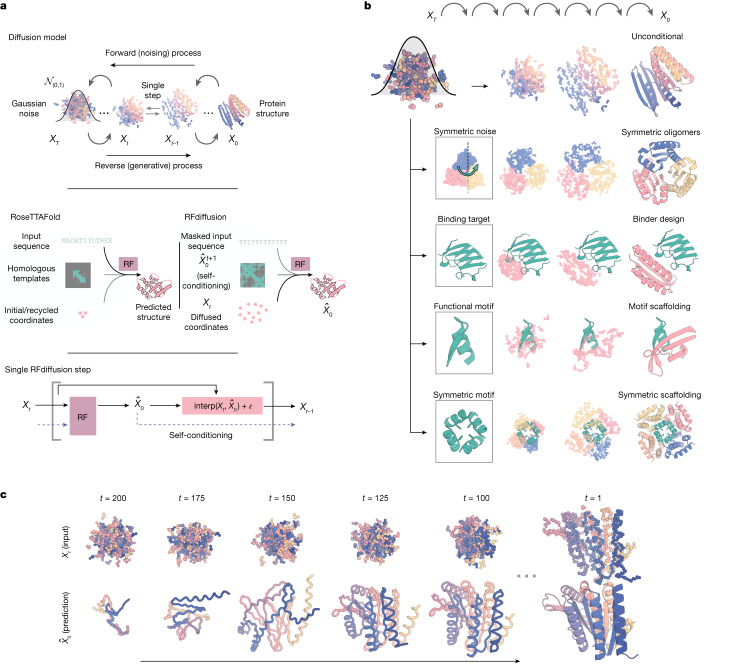
In antibody structure design, AI technologies can predict the binding affinity of antibodies to specific antigens, thereby guiding efficient antibody design. For example, Nobel Prize laureate David Baker's team used generative AI technology to design entirely new antibodies from scratch. This breakthrough has the potential to bring AI-driven protein design into the multi-billion-dollar therapeutic antibody market. Their RFdiffusion tool integrates the powerful capabilities of deep learning with refined biological knowledge, providing an efficient platform for antibody design and optimization. RFdiffusion, based on Generative Adversarial Networks (GANs), trains generative models to create new data that is nearly indistinguishable from real data while training discriminator models to differentiate between real and generated data.
AI-Assisted Antibody Optimization
In sequence optimization, AI helps researchers predict how antibody mutations affect their affinity and specificity, guiding more effective antibody sequence design. For instance, the AntBO framework, developed by Huawei Noah's Ark Lab, efficiently designs the CDRH3 region of antibodies by generating predictions for higher-affinity sequences through Bayesian optimization. AntBO iteratively proposes CDRH3 sequences, evaluates their affinity with Absolut, and adjusts posterior probabilities based on these evaluations. This method generates new CDRH3 sequences with higher affinity while requiring significantly fewer protein designs than traditional methods.
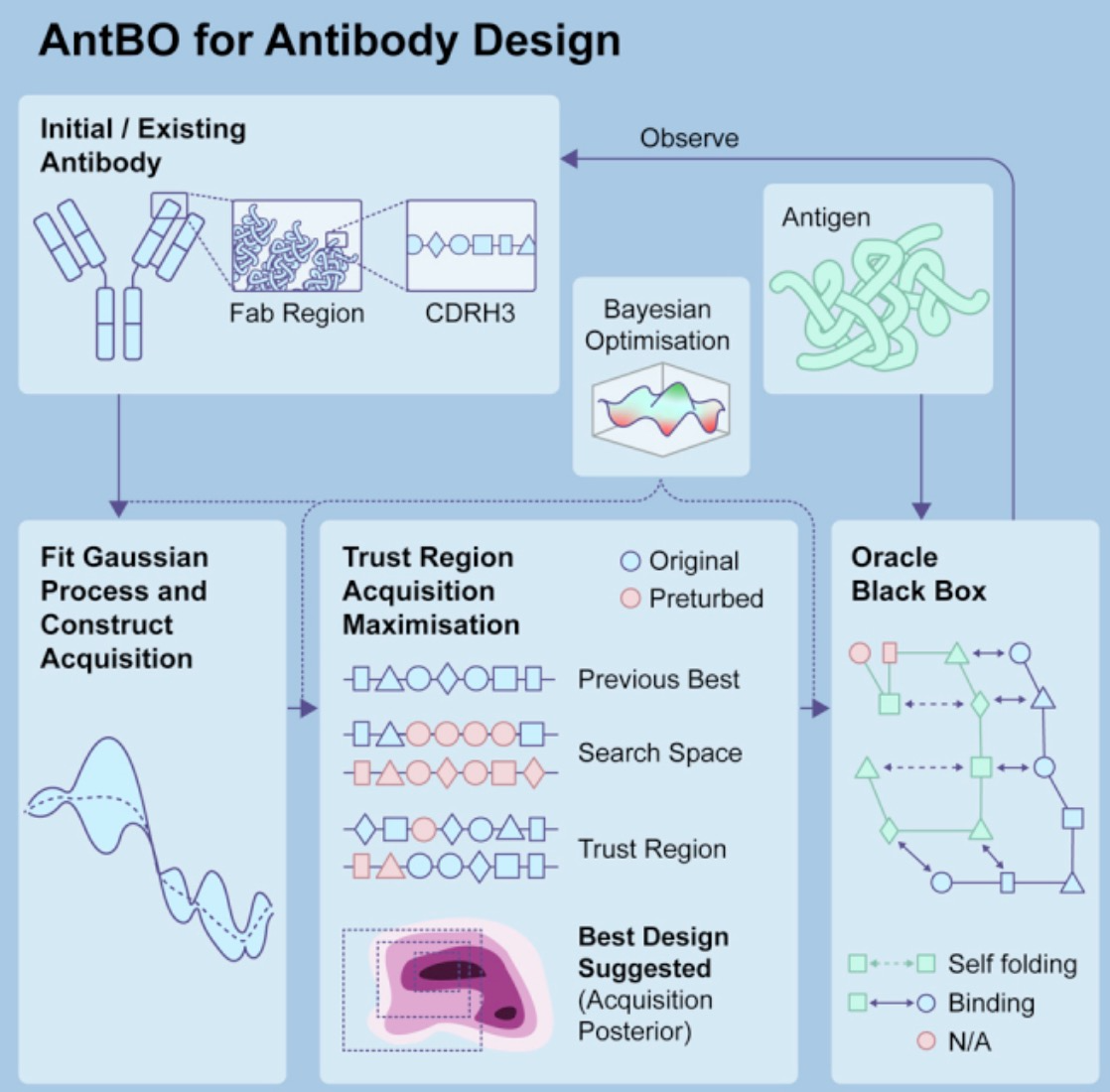
Moreover, AI can analyze vast amounts of antibody sequence data to predict and optimize amino acid sequences, enhancing stability, affinity, and specificity. AI can also quickly predict antibody 3D structures, helping researchers understand antigen-antibody interactions and optimize design strategies. AI learns from historical data, forecasting which design and optimization approaches are most likely to succeed.
AI-Driven Antibody Affinity Improvement
AI technology is revolutionizing antibody affinity improvement. By analyzing large datasets of antibody-antigen interactions, AI identifies design principles that enhance antibody affinity and predicts how mutations influence affinity and specificity, leading to more effective antibody sequence designs.
For example, Absci developed an AI platform that uses high-throughput affinity data to train deep contextual language models. These models accurately predict the binding affinity of unknown antibody sequence variants. This approach combines experimental data with AI predictions, optimizing antibody binding affinity and improving drug development efficiency. Additionally, Absci developed the ACE method, which integrates Fluorescence-Activated Cell Sorting (FACS) with Next-Generation Sequencing (NGS) to generate high-throughput measurements of antibody binding affinity. This method accelerates the screening process, enabling the rapid identification of high-affinity antibody candidates and speeding up the drug development process.
AI-Assisted Antibody Drug Discovery and Development
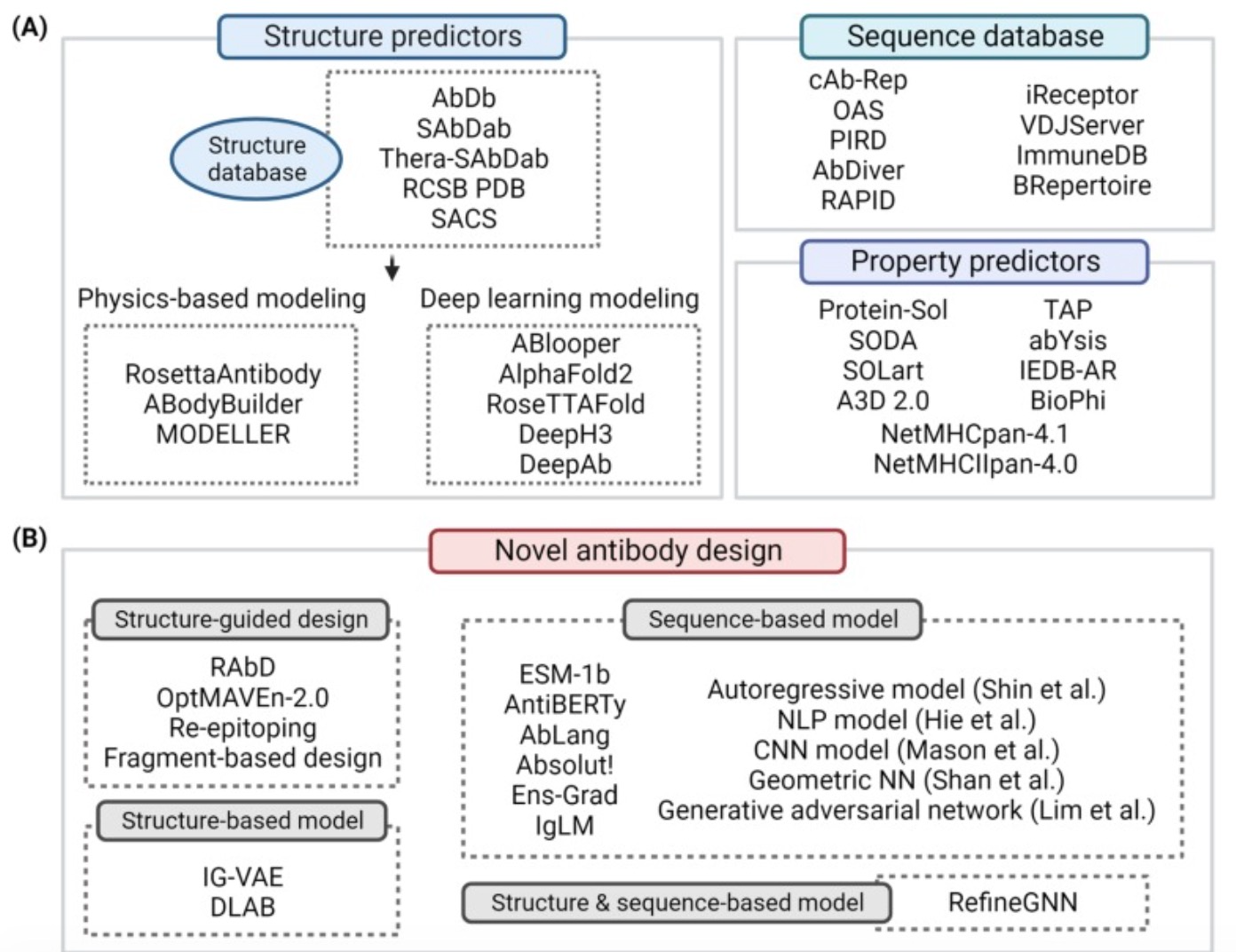
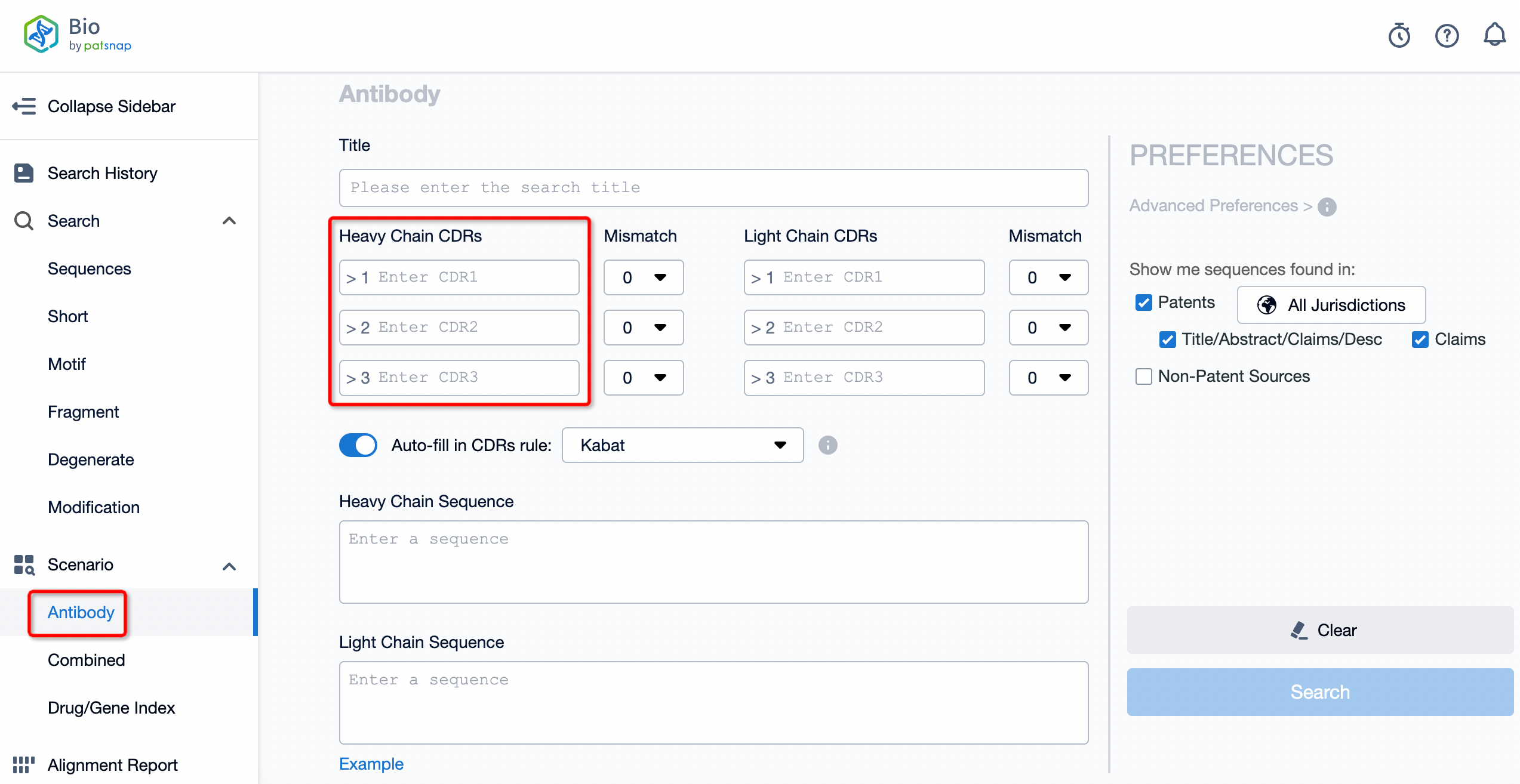
In the development of antibody drugs, AI plays a role beyond design and optimization. It contributes to building antibody sequence/structure databases, predicting antibody performance, and AI-based drug design. For instance, Patsnap Bio provides comprehensive sequence data and advanced alignment algorithms, enabling researchers to quickly search and analyze global similar sequences, helping them manage risk and gather competitive intelligence. The database contains over 300 million sequences, covering a vast amount of patent data, biological journals, and public databases of protein and nucleic acid sequences. This comprehensive dataset forms a solid foundation for antibody drug development.
Patsnap Bio enhances the efficiency of retrieving sequence information by annotating key biological sequences and structural modifications. It also integrates a knowledge graph linking biological sequences, species, drugs, diseases, structures, and functions, providing rich background and deep analysis for drug research. Additionally, it offers multiple search functionalities tailored to researchers’ needs, including CDR searches, multi-sequence searches, patternized sequences, and structural domain searches. Patsnap Bio’s unique sequence search function, supported by a proprietary algorithm and a 45-million sequence library, allows users to search sequences containing operators and degenerate symbols, reducing the risk of missing sequences in freedom-to-operate (FTO) assessments.
AI Impact on Antibody Drug R&D
AI's application in antibody drug R&D significantly speeds up discovery, enhances accuracy, reduces costs, and accelerates drug development. Traditional antibody drug development is labor-intensive, requiring extensive screening and validation. However, AI allows scientists to quickly analyze vast genomic and proteomic datasets to identify promising antibody candidates. Researchers can simulate and predict the functional characteristics of antibodies, such as specificity and toxicity, before synthesis, saving time and resources.
AI platforms also simulate antibody-target interactions, aiding in the design of drugs more likely to be effective against specific diseases. These simulations can also predict an antibody's immunogenicity, reducing the risk of adverse immune responses and improving drug safety. This accelerates the overall drug development cycle, which is critical for addressing rapidly mutating diseases and unmet medical needs.
As AI technology advances, its impact on drug development will continue to grow. In antibody drug research, AI promises to open new therapeutic avenues. By combining advanced machine learning models with high-performance computing, AI will further drive drug discovery. Beyond antibodies, AI will play a crucial role at every stage of drug development, from target identification to post-market surveillance. Especially in antibody therapies, AI’s predictive and data analysis capabilities are expected to reveal novel therapeutic strategies against diseases like cancer, autoimmune disorders, and infectious diseases.
The integration of AI into the biopharmaceutical industry not only accelerates existing processes but also signals the advent of personalized medicine and precision therapies, creating unprecedented pathways to address the world’s toughest health challenges.
Better answers for better bio-innovations!
Validate novelty, eliminate risk, and innovate with confidence using the world’s largest sequence database curated from millions of patent and non-patent sources.
Patsnap Bio helps you turn weeks into minutes with cutting-edge AI-enabled tools built to master the complexities of sequence retrieval and automate IP analysis with precision and ease.
With best-in-class coverage of protein and nucleic acid sequences combined with state-of- the-art search algorithms, you’ll spend less time searching and more time bringing your bio-innovations to market.
Reference:
1. Kim, J., McFee, M., Fang, Q., Abdin, O. & Kim, P. M. Computational and artificial intelligence-based methods for antibody development. Trends Pharmacol Sci 44, 175-189 (2023). https://doi.org:10.1016/j.tips.2022.12.005
2. Watson, J. L. et al. De novo design of protein structure and function with RFdiffusion. Nature 620, 1089-1100 (2023). https://doi.org:10.1038/s41586-023-06415-8
3. Khan, A. et al. Toward real-world automated antibody design with combinatorial Bayesian optimization. Cell Rep Methods 3, 100374 (2023). https://doi.org:10.1016/j.crmeth.2022.100374




