Three Gan & Lee Pharmaceuticals Drugs, GZR18, GZR4, and GZR101, Succeed in Phase 2 Trials
Gan & Lee Pharmaceuticals (Gan & Lee, Shanghai Stock Exchange: 603087) revealed that three drugs they independently developed—the bi-weekly GLP-1 receptor agonist GZR18 injection, the once-weekly basal insulin analog GZR4 injection, and the premixed dual insulin analog GZR101 injection—successfully produced favorable outcomes in three Phase 2 clinical studies conducted on adult Type 2 diabetes (T2D) patients in China. In these trials, the three innovative medications from Gan & Lee exhibited either superior or comparable effectiveness in reducing glycated hemoglobin (HbA1c) levels compared to each of their respective positive comparator drugs.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
GZR18 Injection: A Phase 2 Study Investigating Efficacy and Safety of GZR18 Injection Against Semaglutide (Ozempic®) in Chinese T2D Patients
The Phase 2b clinical study (CTR20232069) is a multicenter, randomized, open-label trial assessing the efficacy and safety of the GZR18 injection in comparison to semaglutide (Ozempic®) among Chinese adults with Type 2 Diabetes who are not maintaining adequate glycemic control through lifestyle changes and/or inconsistent use of anti-diabetic medications, despite being on oral anti-diabetic treatments for at least three months. A total of 264 participants were enrolled from 25 healthcare centers across China. Participants were randomly assigned to receive either bi-weekly injections of GZR18 (12 mg, 18 mg, or 24 mg) or weekly 24 mg GZR18 injections, or 1 mg of semaglutide over 24 weeks, including a dose-escalation phase. The primary objective was to measure the change in HbA1c levels from the beginning after 24 weeks of ongoing treatment. Participants had a diabetes history ranging from 11 to 18 years, with initial HbA1c values ranging between 8.28% and 8.56%.
GZR4 Injection: Phase 2 Study on Efficacy and Safety of GZR4 versus Insulin Degludec (Tresiba®) in Chinese T2D Patients
The Phase 2 study (CTR20232431) was organized as a multicenter, randomized, open-label, parallel-group trial examining the efficacy, tolerability, and safety of once-weekly GZR4 injections compared with daily insulin degludec (Tresiba®) in 83 T2D patients with inadequate glycemic management on oral anti-diabetic medication (Part A) and in 96 T2D patients struggling to control glucose levels with a combination of oral anti-diabetic drugs and basal insulins (Part B).
GZR101 Injection: Phase 2 Study of Efficacy and Safety of GZR101 versus Insulin Degludec/Insulin Aspart (Ryzodeg®) in Chinese T2D Patients
The Phase 2 clinical trial (CTR20232431) is a multicenter, randomized, open-label, parallel-controlled, treat-to-target study. Part A focused on comparing efficacy, safety, and tolerability of daily GZR101 injection versus daily insulin degludec/insulin aspart (Ryzodeg®) over a 16-week period in 62 patients with uncontrolled Type 2 Diabetes on oral anti-diabetic drugs. Part B of the study evaluated the efficacy, safety, and tolerability of GZR101 injection used in conjunction with insulin aspart against twice-daily insulin degludec/insulin aspart (Ryzodeg®) over 16 weeks in 91 patients whose T2D was uncontrolled with basal/premixed insulin.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
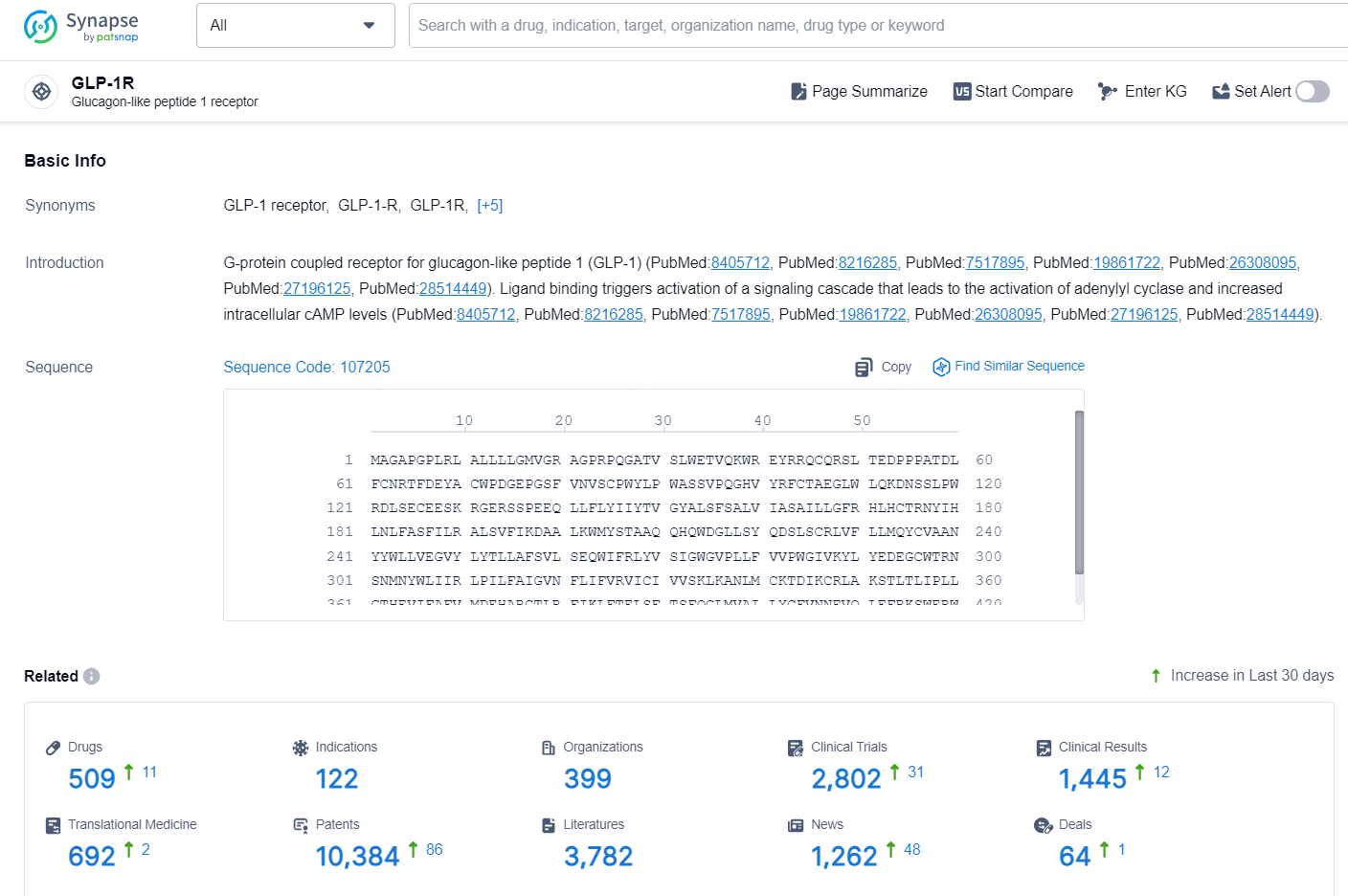
According to the data provided by the Synapse Database, As of October 16, 2024, there are 509 investigational drugs for the GLP-1R targets, including 122 indications, 399 R&D institutions involved, with related clinical trials reaching 2802, and as many as 10384 patents.
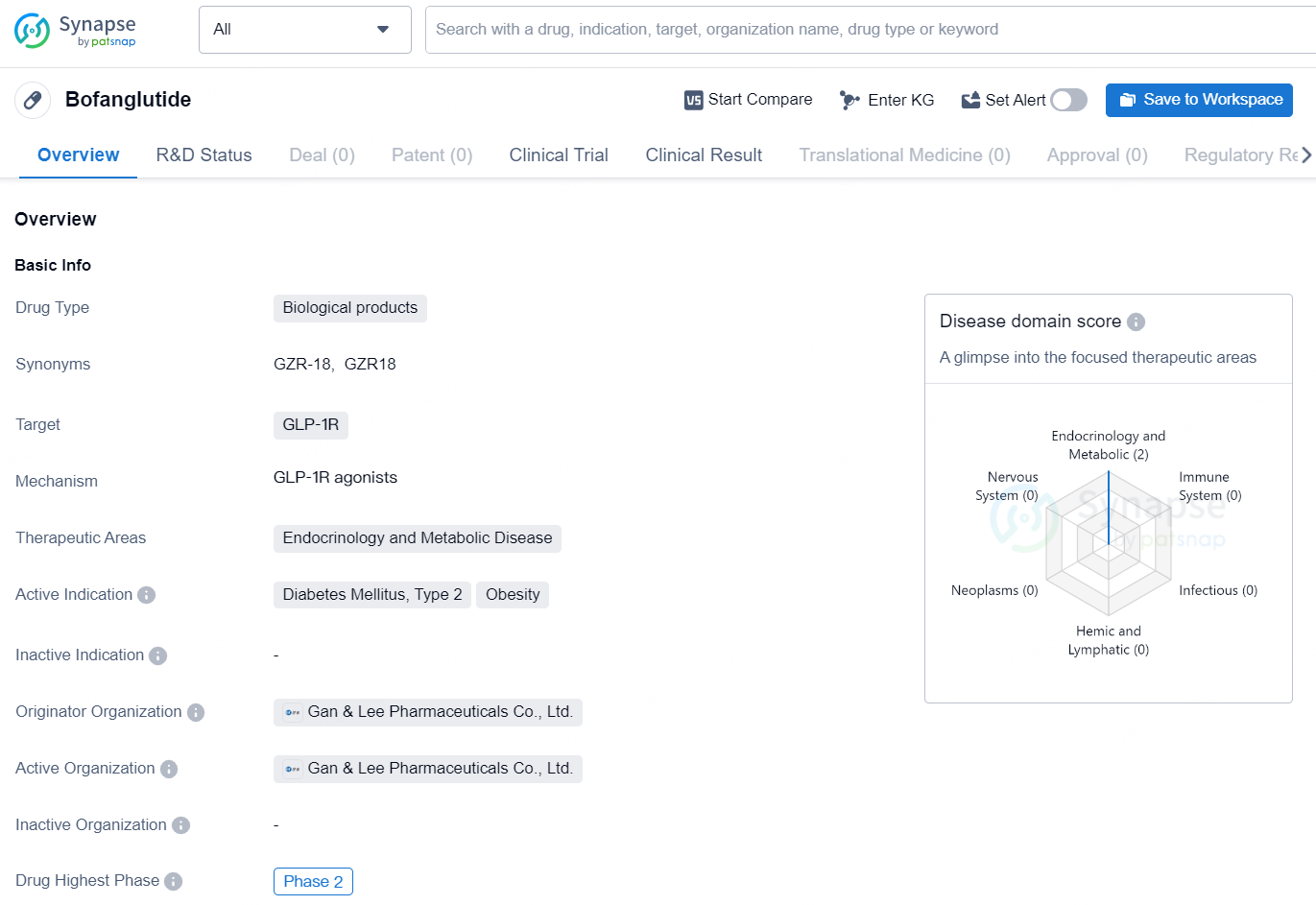
Bofanglutide is a biological product targeting GLP-1R, developed by Gan & Lee Pharmaceuticals Co., Ltd. The drug is primarily intended for therapeutic use in the areas of endocrinology and metabolic disease, with the main focus on treating Diabetes Mellitus, Type 2, and Obesity. As of the latest information available, Bofanglutide has reached Phase 2 clinical trials, indicating that it has shown potential for efficacy and safety in early and mid-stage testing. The highest phase reached for Bofanglutide both globally and in China is Phase 2, suggesting ongoing development and evaluation of the drug's clinical profile in multiple regions.