RUBY's Phase III clinical trial using Jemperli (dostarlimab) and chemotherapy for endometrial cancer has successfully improved patient survival rates
GSK plc has reported encouraging leading findings from an intended analysis of Part 1 of the RUBY/ENGOT-EN6/GOG3031/NSGO phase III study examining Jemperli (dostarlimab) in combination with standard-of-care chemotherapy, then as an individual therapy, in relation to a placebo together with chemotherapy then another placebo, in mature patients afflicted by primary advanced or recurrent endometrial cancer. The study achieved its principal goal of overall survival, unveiling a statistically considerable and clinically relevant advantage for the entire patient group.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In both pre-established subgroups in the trial - those with mismatch repair deficiency/microsatellite instability-high and those proficient in mismatch repair/microsatellite stable - a substantial clinical OS gain was noticed. In the RUBY Part 1 trial, OS stands as one of the two main points of focus. The trial had formerly met its alternate primary point, PFS, with a notable 72% and 36% drop in the probability of disease progression or fatality in the dMMR/MSI-H populace.
Expressing his views on the interim results of Part 1 of the phase III RUBY trial, Hesham Abdullah, the Senior Vice President, Global Head Oncology, R&D, GSK, stated: “The results today confirm that the combination of dostarlimab and chemotherapy is the singular immuno-therapeutic blend to exhibit a survival advantage in this wider patient segment in this therapeutic context. We eagerly await disseminating this analysis' in-depth results to regulatory bodies and the wider scientific spectrum.”
As of now, Jemperli has regulatory sanctions for a specific subset of patients suffering from endometrial cancer, this was based on the earlier announced favourable results for the main endpoint of PFS in RUBY trial’s Part 1.
Jemperli got the green light from the FDA in July 2023, when used with carboplatin and paclitaxel, then applied as a sole agent for treating mature patients having primary progressive or repeated endometrial cancer, an FDA-sanctioned test verified it as mismatch repair deficient or microsatellite instability-high. The United Kingdom has also approved Jemperli.
Jemperli’s acting formula includes a programmed death receptor-1 (PD-1)-inhibiting antibody that merges with the PD-1 receptor, impeding its interaction with the PD-1 ligands PD-L1 and PD-L2. It was approved in the UK in October 2023 along with platinum-based chemotherapy for treating adult patients with primary advanced or recurring dMMR/MSI-H endometrial cancer who are eligible for systematic treatment.
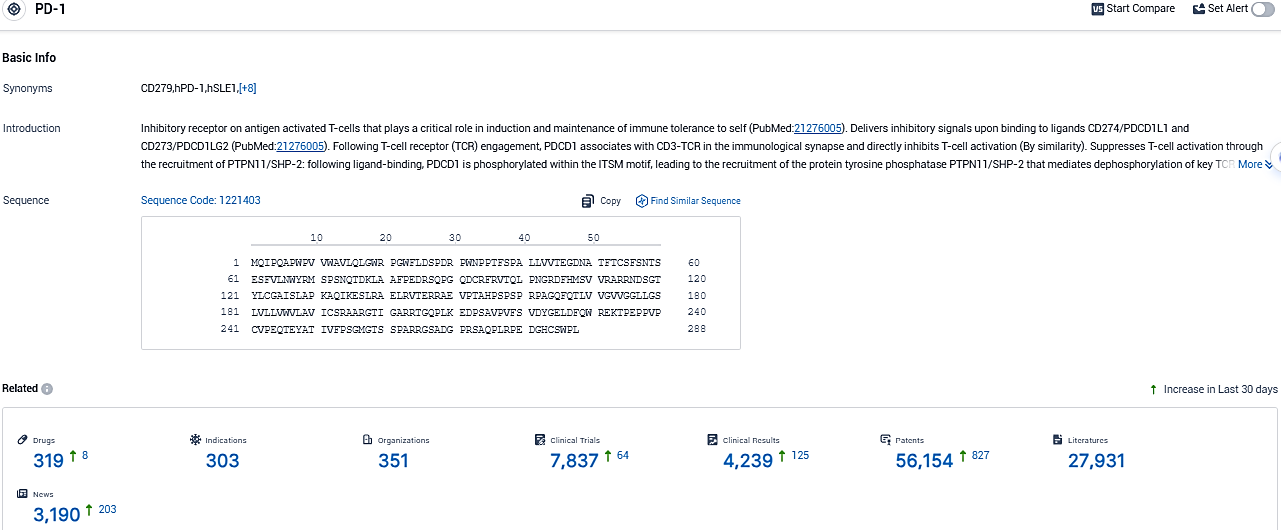
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 2, 2023, there are 319 investigational drugs for the PD-1 target, including 303 indications, 351 R&D institutions involved, with related clinical trials reaching 7837, and as many as 3190 patents.
Jemperli is administered in the United States in conjunction with carboplatin and paclitaxel. Following this, Jemperli is given exclusively for the purpose of managing adults with primary progressive or relapsed endometrial cancer, which is identified as mismatch repair deficient through a diagnostic tool that has received FDA approval or is characterized as high in microsatellite instability. Jemperli is also employed independently for adult individuals afflicted with mismatch repair-deficient recurrent or progressive endometrial cancer.