Samsung Bioepis collaborates with Sandoz to market Ustekinumab Biosimilar Candidate
Samsung Bioepis Co., Ltd. disclosed it has struck a commercialization pact with Sandoz regarding SB17, a potential biosimilar to Stelarai(ustekinumab). This signifies progress in enhancing the availability of Samsung Bioepis' immunology collection in the US, Canada, EEA, Switzerland, and the UK.
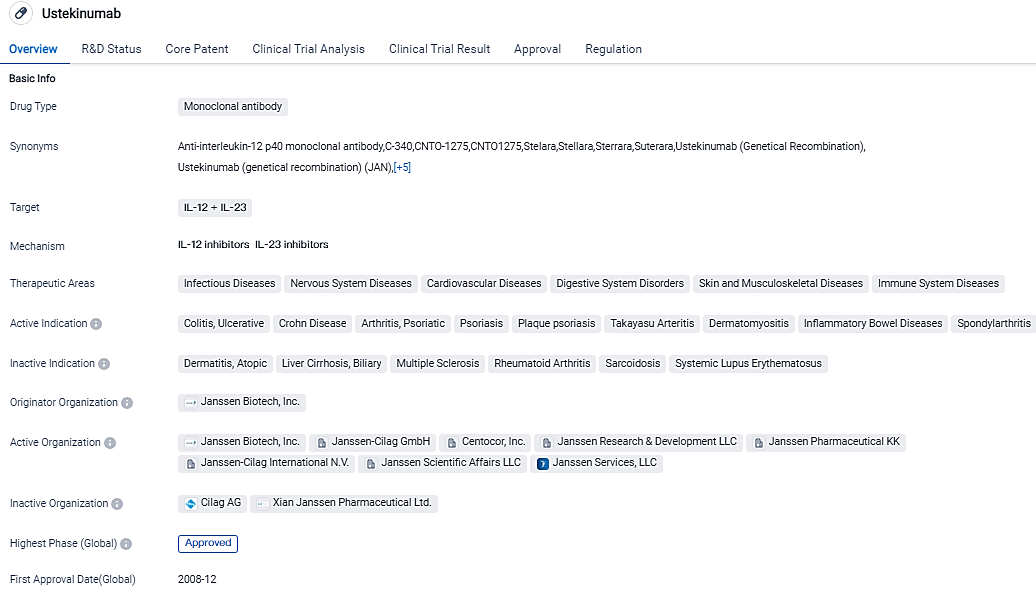
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"This partnership is a reflection of Samsung Bioepis' commendable performance in the realm of immunology, indicating the potential impact our biosimilars could have in broadening the accessibility to biologic medicines," said Sang-Jin Pak, senior Vice President and Chief of the Commercial Division, at Samsung Bioepis.
B17, a proposed biosimilar of Stelara, takes the fourth place in Samsung Bioepis' immunology pipeline, coming after SB4 (etanercept), SB2 (infliximab) and SB5 (adalimumab). Samsung Bioepis boast a strong record of more than five years, having delivered over 48 million units of immunology biosimilars to almost 40 markets worldwide.
In March, Samsung Bioepis showcased results from a Phase 1 clinical study of SB17 at the 2023 AAD Annual Meeting, which confirmed kinetic equivalence and similar safety, tolerability, and immunogenicity profiles of SB17 and reference ustekinumab. The results of SB17 Phase 3 clinical study, which ended in December 2022, will be revealed at a medical congress happening within the year.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
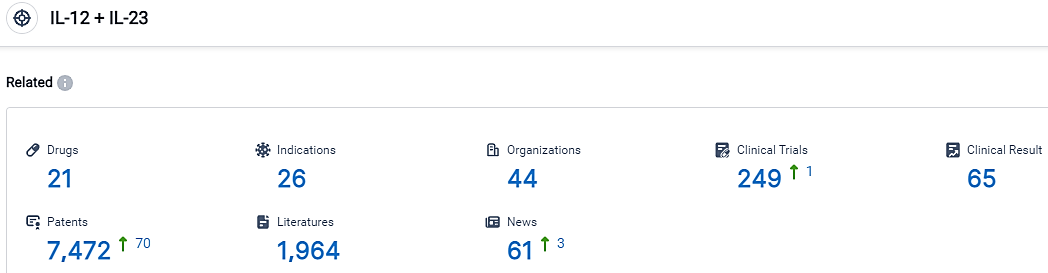
According to the data provided by the Synapse Database, As of September 13, 2023, there are 21 investigational drugs for the IL-12 and IL-23 target, including 26 applicable indications,44 R&D institutions involved, with related clinical trials reaching 249,and as many as 7472 patents.
Ustekinumab's approval in various therapeutic areas and its designation as an orphan drug highlight its potential as a valuable treatment option for patients suffering from a range of diseases. Its mechanism of action and proven efficacy make it a promising drug in the field of biomedicine.