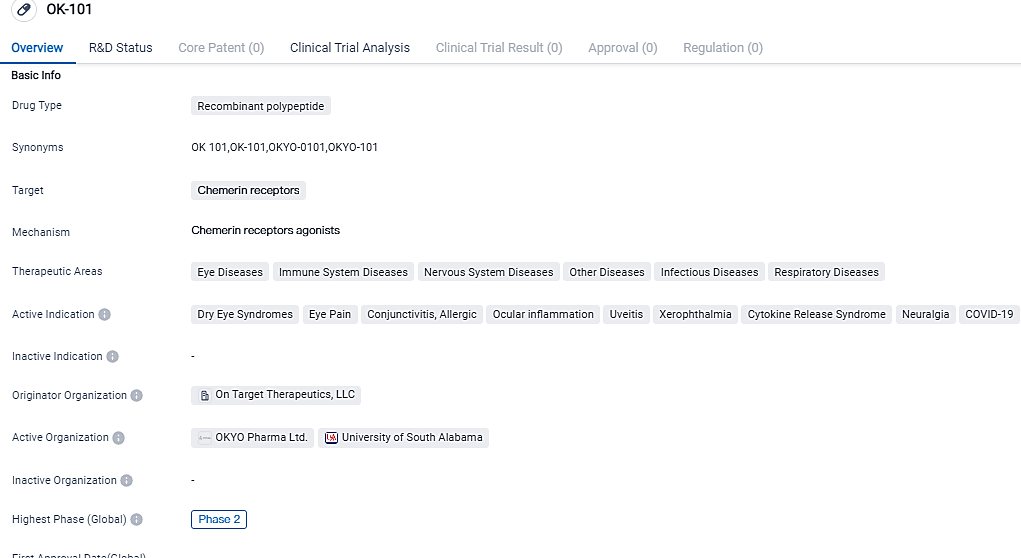
OKYO Pharma Concludes Patient Recruitment for Phase 2 Clinical Trial of OK-101 to Dry Eye Syndrome
OKYO Pharma Limited, a clinical stage biopharmaceutical firm developing innovative treatments for inflammatory dry eye disease, a market worth several billion dollars, and neuropathic corneal distress, a harsh eye condition that lacks FDA-approved therapy, is delighted to share it has now fully enrolled its quota of patients for the randomised section of the Phase 2 clinical trial. The study, which is double-masked and placebo-controlled, is intended to test the topical ocular OK-101 as a treatment for DED. A sum of 240 patients have registered for the study thus far.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"This trial began in May of this year, we are satisfied with the swift enrollment rate in our trial, orchestrated by our clinical development collaborator, Ora Inc., a global authority in dry eye clinical investigation,” stated Gary S. Jacob, Ph.D., the CEO of OKYO Pharma. “With 240 participants now in the trial, we foresee the last-patient last-visit happening in the final week of November 2023, meanwhile, we anticipate to disclose the top-line data by December 2023.”
“Phase 2 clinical trial is a crucial step in the progression of OK-101, conducting an assessment of its safety, effectiveness, and tolerability in the cohort of 240 DED patients that comprise the study,” cited Raj Patil, Ph.D., the CSO of OKYO Pharma. “Our dedication remains unwavering to ascertain the potential of this pharmaceutic for the treatment of the vast number of individuals presently battling DED."
DED is a common condition that occurs when one’s tears are unable to adequately lubricate the eyes. The condition influences roughly 49 million individuals solely in the United States and has posed challenges in diagnosis and therapeutic intervention due to the condition's multifactorial characteristics. Numerous factors could contribute to this condition, including age, gender, specific health conditions, decreased tear secretion and tear film malfunction. Generally, tear film instability results in ocular surface inflammation and damage, and pain.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
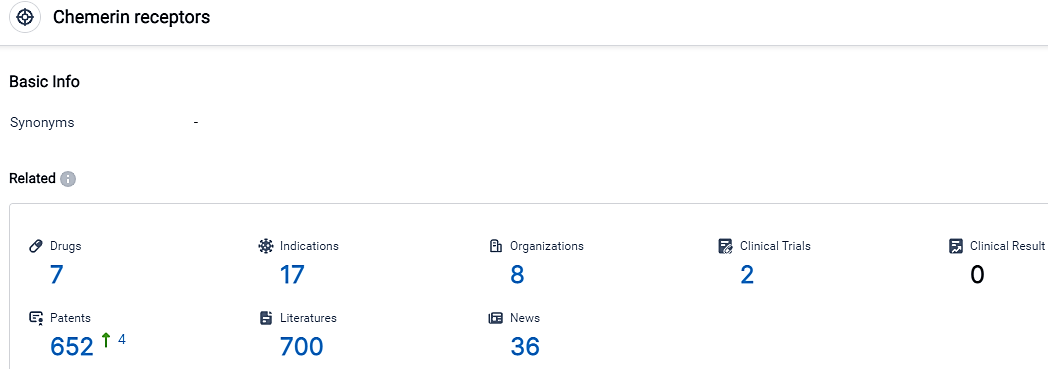
According to the data provided by the Synapse Database, As of September 13, 2023, there are 7 investigational drugs for the Chemerin receptors, including 17 applicable indications, 8 R&D institutions involved, with related clinical trials reaching 2,and as many as 652 patents.
OK-101 has been shown to produce anti-inflammatory and pain-reducing activities in mouse models of dry eye disease and corneal neuropathic pain, respectively, and is designed to combat washout through the inclusion of the lipid ‘anchor’ contained in the drug molecule to enhance the residence time of OK-101 within the ocular environment. OK-101 is currently in a Phase 2, multi-center, double-masked, placebo-controlled trial to treat dry eye disease.