Sandoz introduces Hyrimoz® (adalimumab) high-dose variant in Europe to enhance patient treatment
Sandoz, the worldwide pioneer in generic and biosimilar medication, today publicizes the introduction of Hyrimoz® (adalimumab) without citrate high concentration version in Europe. The medication will gradually become accessible to patients throughout European nations, the process commences today.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"Living with chronic inflammatory diseases can severely disrupt everyday life," said Rebecca Guntern, Europe President of Sandoz. “The roll out of Hyrimoz® HCF in Europe marks an important step in providing an alternative therapy for those in need, reflecting our ongoing dedication to broadening the availability of high-standard treatments."
Hyrimoz® citrate-free HCF, an improved formulation of the existing Hyrimoz® 50 mg/mL, reduces the injection volume by half, which may lessen the injection frequency for patients requiring an 80 mg/mL or higher dose. The HCF formulation is applied with the familiar Hyrimoz® SensoReady® pen, aiming to optimise the patient experience while keeping it recognisable.
The introduction of Hyrimoz ® HCF fortifies Sandoz's biosimilar selection in the field of immunology, which includes Erelzi® (biosimilar etanercept), Zessly® (biosimilar infliximab), and Rixathon® (biosimilar rituximab, including for the treatment of rheumatoid arthritis). The Hyrimoz® citrate-free HCF debuted in the US market in July 2023.
Dedicated to enabling millions of patients to access vital and possibly life-altering biologic treatments sustainably and economically, Sandoz operates in various fields including immunology, oncology, supportive care, and endocrinology. The company boasts a foremost international portfolio of eight marketed biosimilars, along with 25 additional assets at various developmental stages.
After launching the first European biosimilar in 2006, Sandoz has played a major part in facilitating early and widespread patient access to transformative treatments, leading to considerable healthcare cost savings and driving competition that spurs additional innovations.
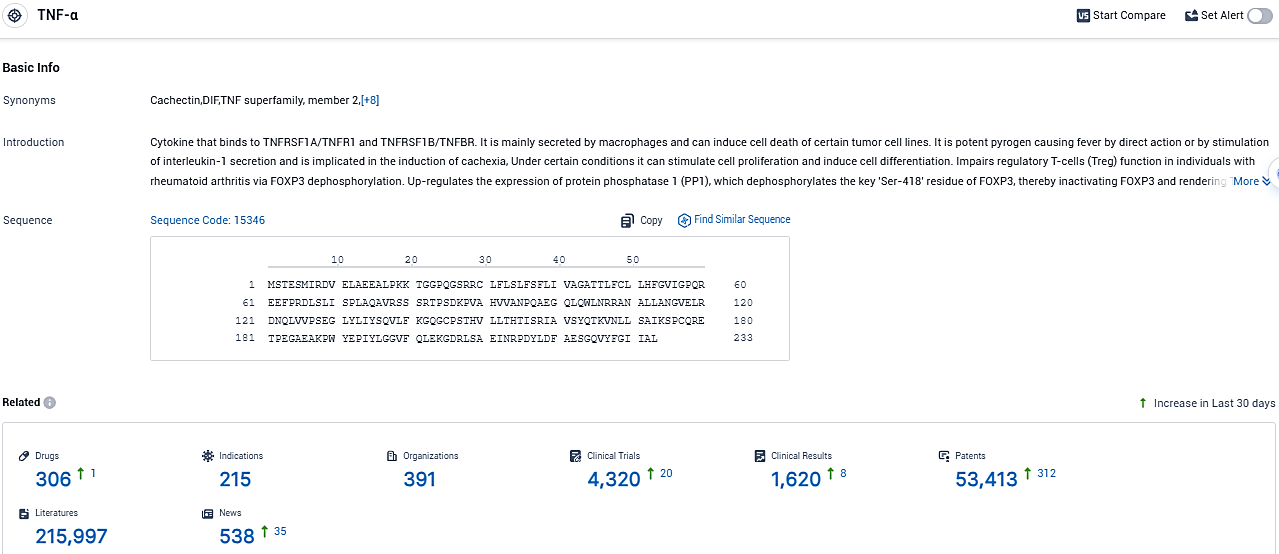
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 28, 2023, there are 306 investigational drugs for the TNF-α target, including 215 indications, 391 R&D institutions involved, with related clinical trials reaching 4320, and as many as 53413 patents.
Adalimumab is a human immunoglobulin G1 monoclonal antibody targeting tumor necrosis factor alpha (TNF-α). The adalimumab reference medicine (Humira®*) was first approved with an adalimumab concentration of 50 mg/mL.1 In 2015, the EMA and US FDA approved Humira® HCF, which contains adalimumab at a concentration of 100 mg/mL.