Sanofi and Orano collaborate to create advanced radioligand therapies
Sanofi has partnered with Orano Med, a branch of the Orano Group that leads in the advancement of targeted alpha therapies for cancer treatment, to merge their knowledge in combating rare tumors and to expedite the development of advanced radioligand therapies.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Leveraging the knowledge and radioligand development capabilities of Orano Med, Sanofi and Orano will collaborate to create a new entity that will operate under the Orano Med brand. This organization will concentrate on the discovery, design, and clinical advancement of next-generation radioligand therapies (RLTs) utilizing lead-212 (212Pb) alpha-emitting isotopes. This partnership comes in response to Sanofi’s recent announcement regarding an exclusive licensing deal with Orano Med and RadioMedix to promote radioligand therapies (RLTs) aimed at rare cancers, particularly focusing on a late-stage program, AlphaMedix™.
Targeted alpha therapy is based on a straightforward principle: it leverages biological vectors’ ability to hone in on cancer cells while utilizing the short-range, highly powerful destructive effects of alpha-emitting radioisotopes. The vector facilitates the delivery of the radioisotope directly to the cancer cells that express a specific marker, even in cases where they have disseminated throughout the body. This distinctive mode of action aims to effectively damage or eliminate targeted cancer cells while minimizing harm to adjacent healthy tissues. This innovative precision medicine strategy seeks to transform the standard treatment protocols for certain rare cancers, with the intent of prolonging patient longevity and enhancing their quality of life.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
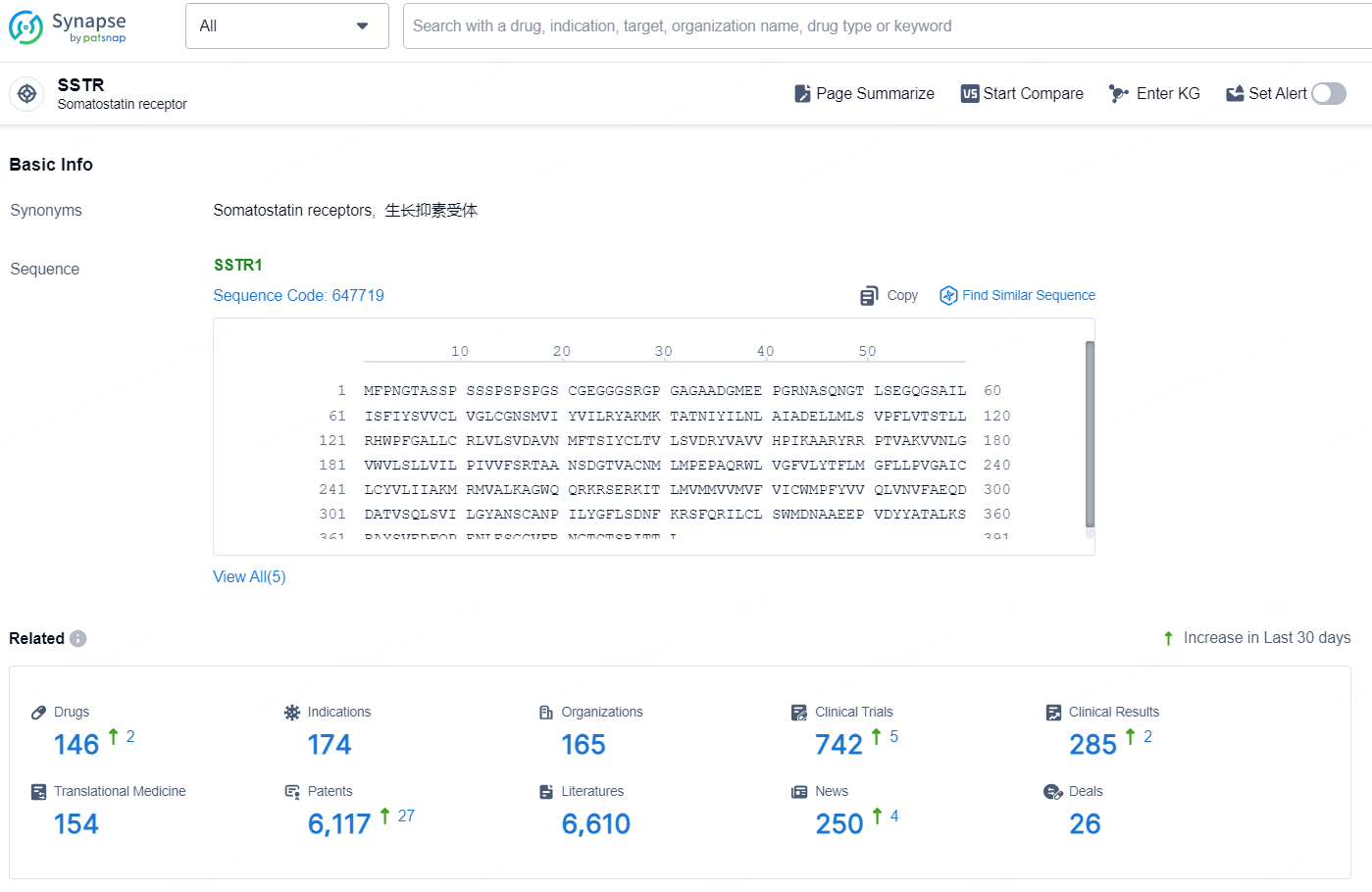
According to the data provided by the Synapse Chemical, As of October 18, 2024, there are 146 investigational drug for the SSTR target, including 174 indications, 165 R&D institutions involved, with related clinical trials reaching 742, and as many as 6117 patents.
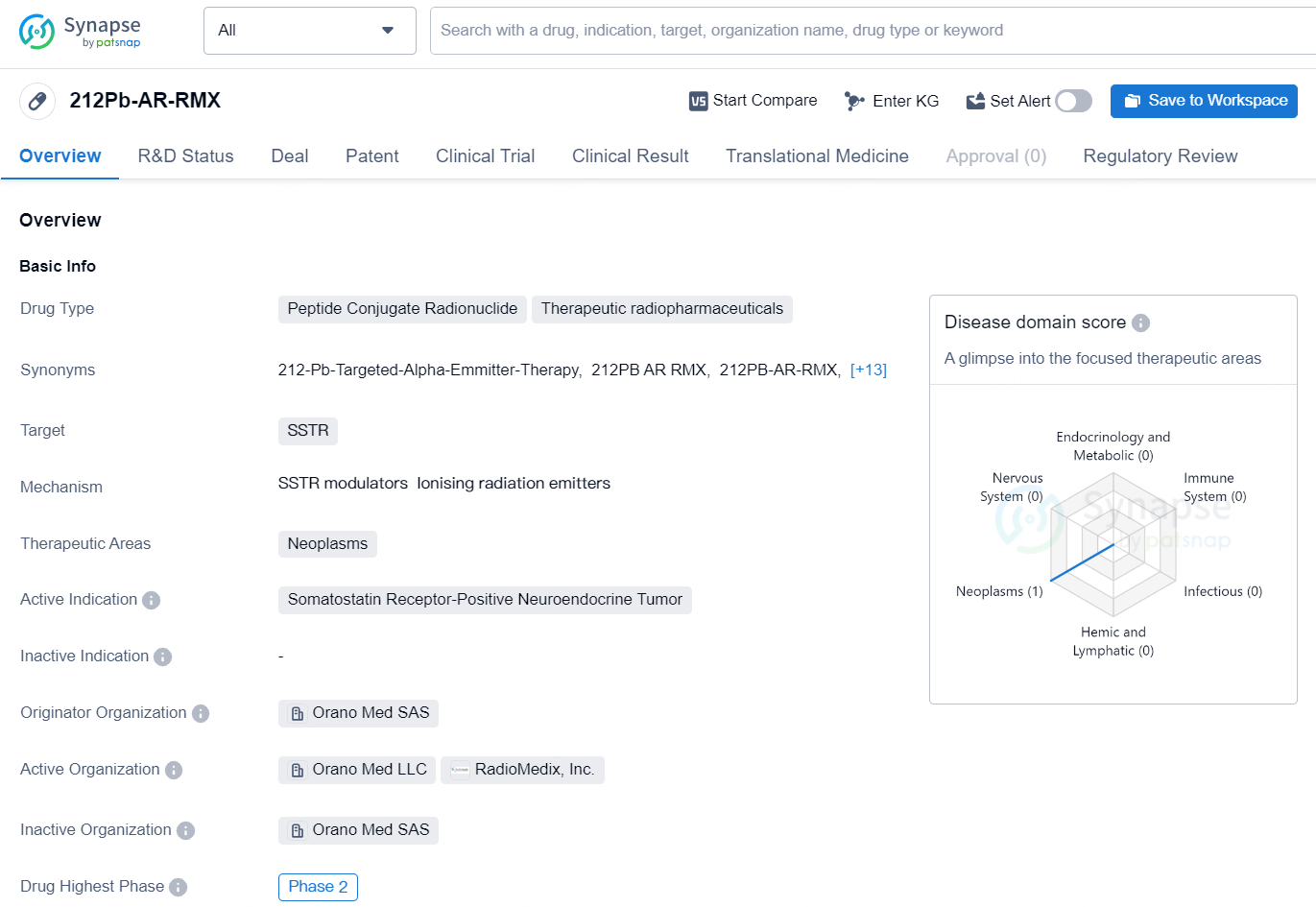
The drug 212Pb-AR-RMX is a peptide conjugate radionuclide classified as a therapeutic radiopharmaceutical. It targets SSTR and is primarily used in the treatment of neoplasms, specifically somatostatin receptor-positive neuroendocrine tumors. Originating from Orano Med SAS, the drug has reached the highest global developmental phase of Phase 2.