uniQure Begins First Patient Treatment in Phase I/II Trial of AMT-162 for SOD1-ALS
uniQure N.V. (NASDAQ: QURE), a pioneering company in the field of gene therapy, dedicated to developing groundbreaking treatments for patients with urgent medical needs, has announced that the initial participant has received a dose in the Phase I/II clinical study of AMT-162, targeting amyotrophic lateral sclerosis (ALS) linked to mutations in the superoxide dismutase 1 (SOD1) gene. This condition represents a rare, inherited, and progressive disorder affecting motor neurons. The EPISOD1 study, a Phase I/II open-label, multi-center trial, is taking place in the United States and involves three groups with escalating doses to evaluate the safety, tolerability, and preliminary indications of efficacy of AMT-162 in SOD1-ALS patients.
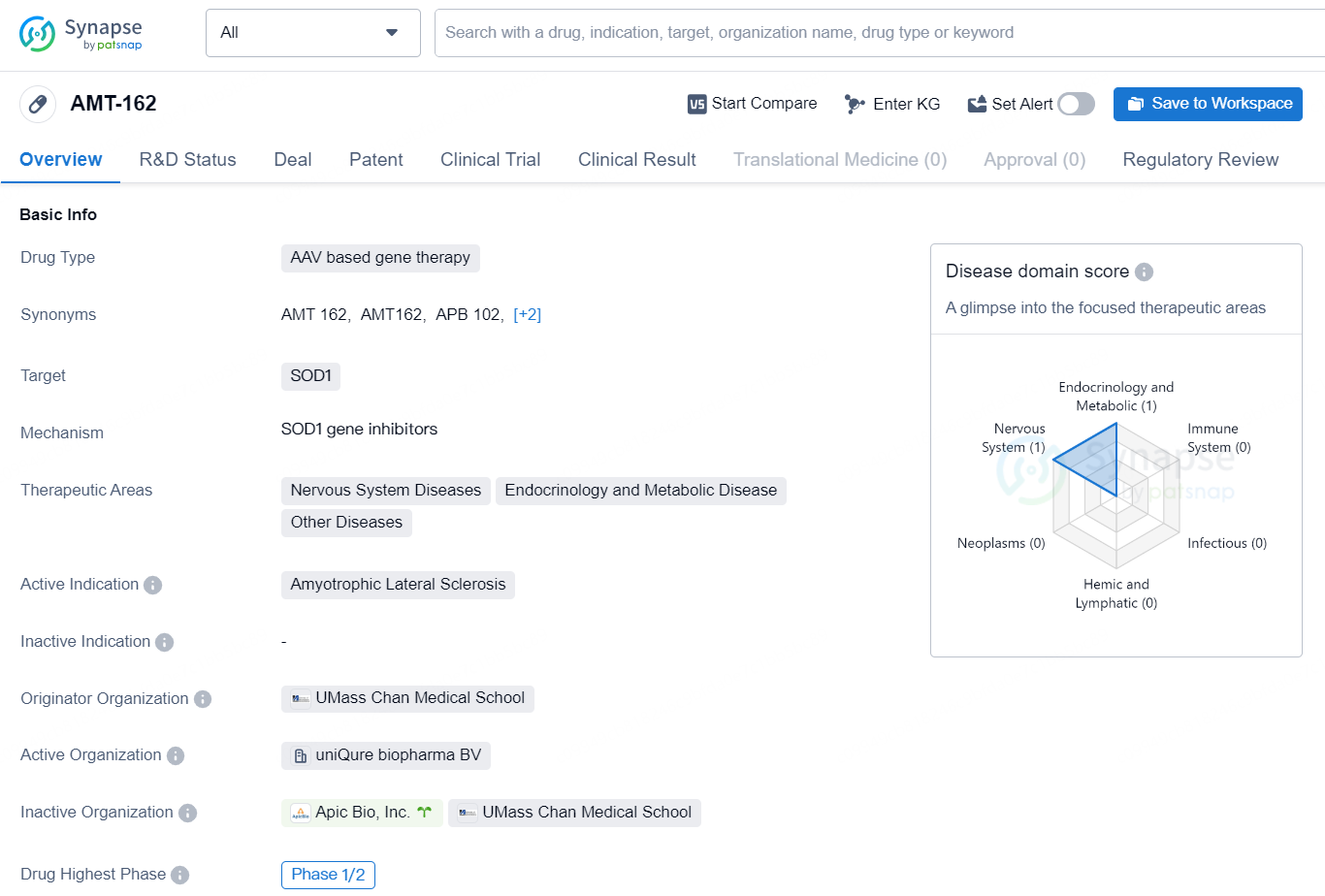
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"We are excited to share that the initial patient has been dosed with AMT-162, our experimental gene therapy designed for SOD1-ALS, a severe, progressive, and terminal illness," said Walid Abi-Saab, M.D., chief medical officer at uniQure. "This trial initiates the progression of our third gene therapy project into clinical trials within this framework, aligning with our objective to quickly produce proof-of-concept data utilizing well-recognized biomarkers to expedite treatment availability for patients. We are optimistic that our innovative AAV-based gene therapy candidate offers the ease of a single dose with the potential for a unique and effective response required for this devastating condition."
AMT-162 is an experimental AAVrh10-based gene therapy that deploys a miRNA aimed at reducing the expression of the altered SOD1 protein. Individuals with SOD1-ALS produce a misfolded SOD1 protein, which is harmful to motor neurons. This results in neuron deterioration that eventually leads to muscle weakness, functional decline, and death. AMT-162 offers a promising one-time, intrathecal method to decelerate or stop the advancement of SOD1-ALS. The U.S. Food and Drug Administration has granted AMT-162 both Orphan Drug designation and Fast Track status.
The EPISOD1 Phase I/II clinical study of AMT-162 will take place in the United States. This multicenter, open-label study involves three groups with a maximum of four patients each, who will receive a brief immunosuppression therapy around the time of intrathecal AMT-162 delivery. The study will investigate the safety and tolerability of AMT-162 while assessing preliminary efficacy signs through neurofilament light chain, a neuronal damage biomarker, and SOD1 protein measurements. Currently, there are four operational sites in the U.S., with intentions to establish seven more sites by early 2025. More information can be found at www.clinicaltrials.gov (NCT06100276).
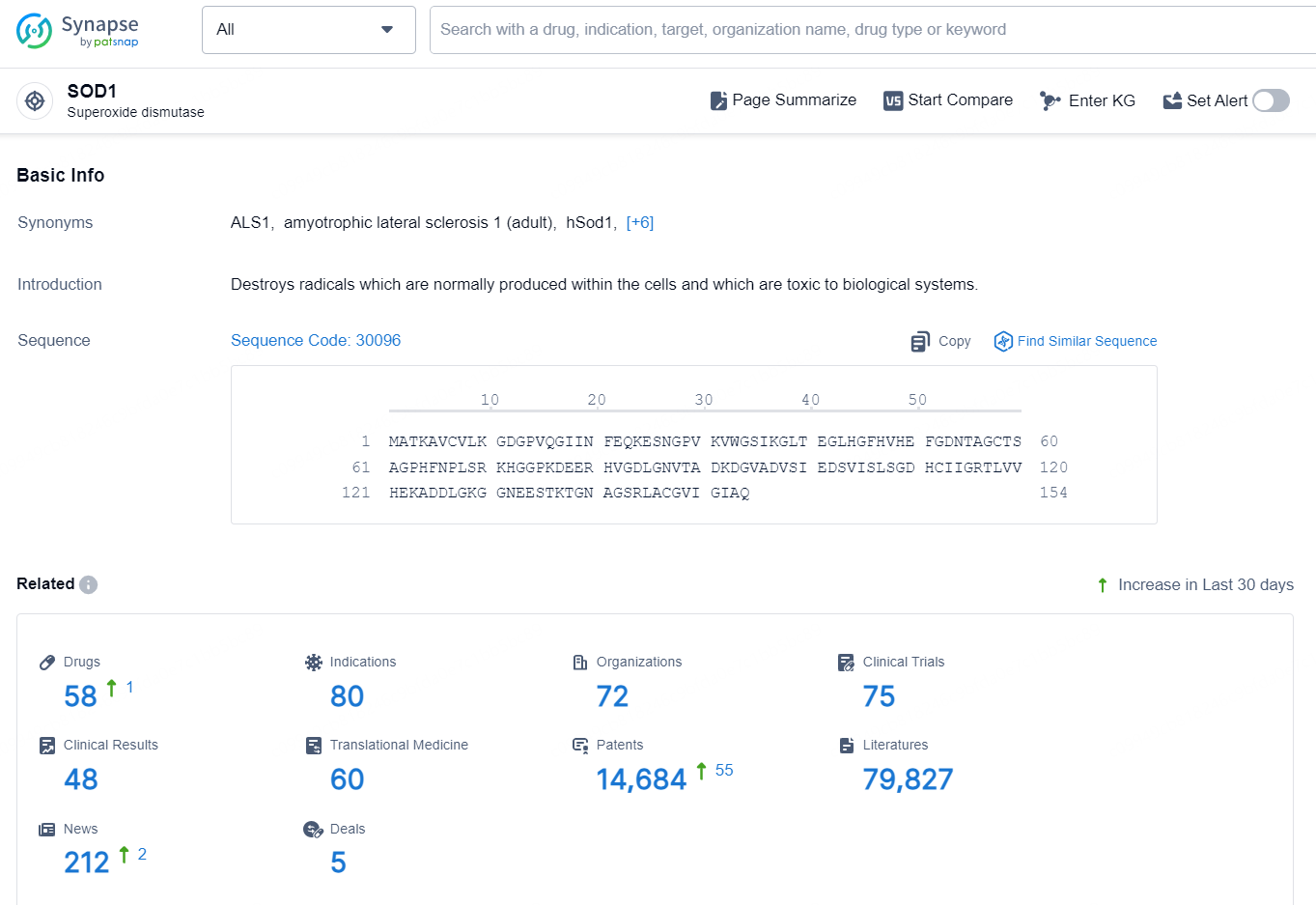
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 16, 2024, there are 58 investigational drugs for the SOD1 targets, including 80 indications, 72 R&D institutions involved, with related clinical trials reaching 75, and as many as 14684 patents.
AMT-162 is an AAV-based gene therapy drug that targets SOD1. It is developed for the treatment of Amyotrophic Lateral Sclerosis (ALS) and falls within the therapeutic areas of Nervous System Diseases, Endocrinology and Metabolic Disease, and Other Diseases. The drug is in the highest phase of development at Phase 1/2, indicating that it is still undergoing clinical trials to assess its safety and efficacy.