Roche's Tecentriq® with ENHANZE® Tech Gets EU Clearance for Subcutaneous Use in Cancer Therapy
Halozyme Therapeutics, Inc. divulged the recent sanction from the European Commission, bestowed upon Roche, for their subcutaneous version of Tecentriq® SC (atezolizumab), which is now blended with ENHANZE®, a unique formulation incorporating Halozyme's patented rHuPH20, a recombinant enzyme derived from human hyaluronidase. This recent endorsement spans the entirety of Tecentriq® IV's previously sanctioned uses and marks a pioneering milestone in the EU for a PD-(L)1 class oncology treatment designed for subcutaneous administration.
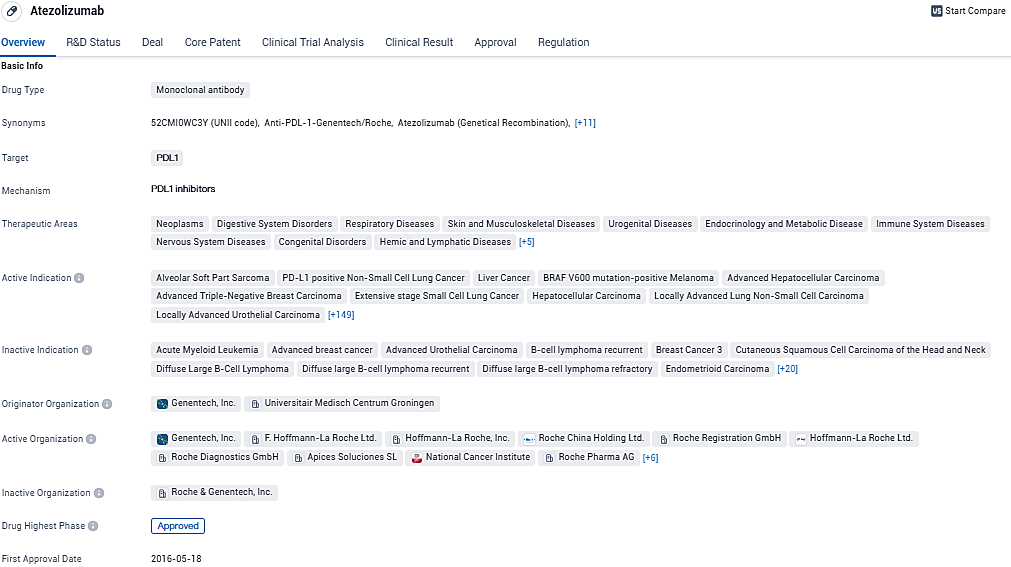
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Tecentriq® SC streamlines administration to roughly a brief 7-minute period, a significant reduction from the traditional intravenous (IV) drip that generally lasts from 30 up to 60 minutes. Furthermore, the formulation can be delivered by a medical professional in various settings beyond the hospital, such as local health centers or even the patient's residence, with adherence to the specific country's health regulations and system capabilities.
Dr. Helen Torley, at the helm as president and CEO of Halozyme, highlighted the significance of Tecentriq SC being the inaugural subcutaneous PD-(L)1 inhibitor for cancer available in Europe. "Tecentriq SC is poised to revolutionize the management of cancer therapies, rendering a more seamless and efficient experience for both patients and healthcare providers. This breakthrough has the potential to alleviate pressures on our stretched healthcare systems," she stated.
The European Commission granted its endorsement based on the foundational outcomes derived from the Phase IB/III IMscin001 trial. The study indicated that the subcutaneous delivery of Tecentriq® achieved blood concentration levels on par with the intravenous approach. It further established a compatibility in safety and effectiveness between the two formulations. Noteworthy in the study was the consensus among 90% of the medical professionals who found the SC version convenient to administer, and 75% acknowledged the time-saving advantages for medical staff in comparison to the traditional IV administration.
Halozyme epitomizes innovation in the biopharmaceutical industry by developing pioneering solutions aimed at enhancing patient experiences and therapeutic outcomes for both new and existing treatments. Through its patented ENHANZE® technology, Halozyme has forged partnerships with industry giants such as Roche, Takeda, Pfizer, AbbVie, Eli Lilly, Bristol Myers Squibb, Alexion, argenx, Horizon Therapeutics, ViiV Healthcare, Chugai Pharmaceutical, and Acumen Pharmaceuticals.
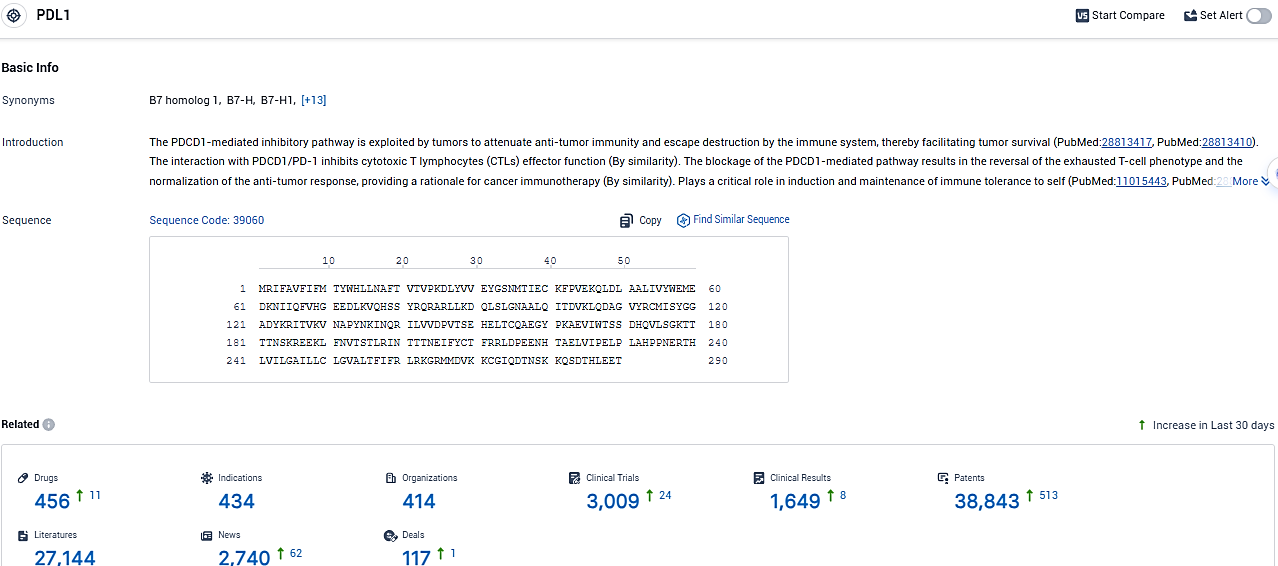
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of January 19, 2024, there are 456 investigational drugs for the PDL1 target, including 434 indications, 414 R&D institutions involved, with related clinical trials reaching 3009, and as many as 38843 patents.
Tecentriq® SC is a monoclonal antibody drug that targets PDL1 and is used in the treatment of various diseases, primarily in the field of biomedicine. It has shown efficacy in treating different types of cancers and has received approvals in the United States and China. The drug has undergone extensive clinical trials and has been granted various regulatory designations.