Scholar Rock Receives FDA Approval for Phase 2 Obesity Trial with Apitegromab
Scholar Rock, an advanced-stage biotech entity dedicated to the development of novel therapies for conditions like spinal muscular atrophy, disorders related to heart health and metabolism, and various critical illnesses with protein growth factors as key components, recently declared approval by the U.S. Food and Drug Administration for the firm's exploratory new pharmaceutical filing. This clearance paves the way for a Phase 2 trial aimed at evaluating the efficacy of apitegromab in managing overweight individuals who are currently on treatment with a GLP-1 receptor stimulant.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The upcoming Stage 2 clinical study is designed as a placebo-controlled, blinded, and randomized trial conducted across various sites. This research aims to determine the impact of the selective myostatin-blocking agent apitegromab. The study will focus on its capability to maintain lean body tissue in combination with a GLP-1 receptor agonist (GLP-1 RA) in individuals who are obese or have excess weight.
The launch of this research is scheduled for the latter half of 2024, with expectations to receive outcomes from the apitegromab Stage 2 study by the middle of 2025. Concurrently, the biopharmaceutical company, Scholar Rock, is advancing the development of SRK-439, an innovative compound targeting myostatin specifically for battling obesity. The enterprise anticipates submitting an Investigational New Drug (IND) application for SRK-439 in 2025.
Scholar Rock's head, Jay Backstrom, M.D., MPH, commented on the U.S. Food and Drug Administration's (FDA) green light for the IND regarding apitegromab for obesity research. As per his statement, this step allows them to investigate the potential of their specially designed myostatin inhibitor to conserve lean muscle tissue. Moreover, it opens up the possibility to evaluate the safety profile and acceptability when used alongside a GLP-1 RA.
Additionally, Dr. Backstrom remarked, the IND paves the way to further refine their strategy for treating metabolic conditions with SRK-439, which is still in the preclinical phase and is being tailored as a selective myostatin inhibitor. He emphasized their eagerness to begin the Stage 2 exploratory trial with apitegromab to confirm the unique strategy that precisely targets pro- and latent myostatin, with the aim of maintaining muscle integrity.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
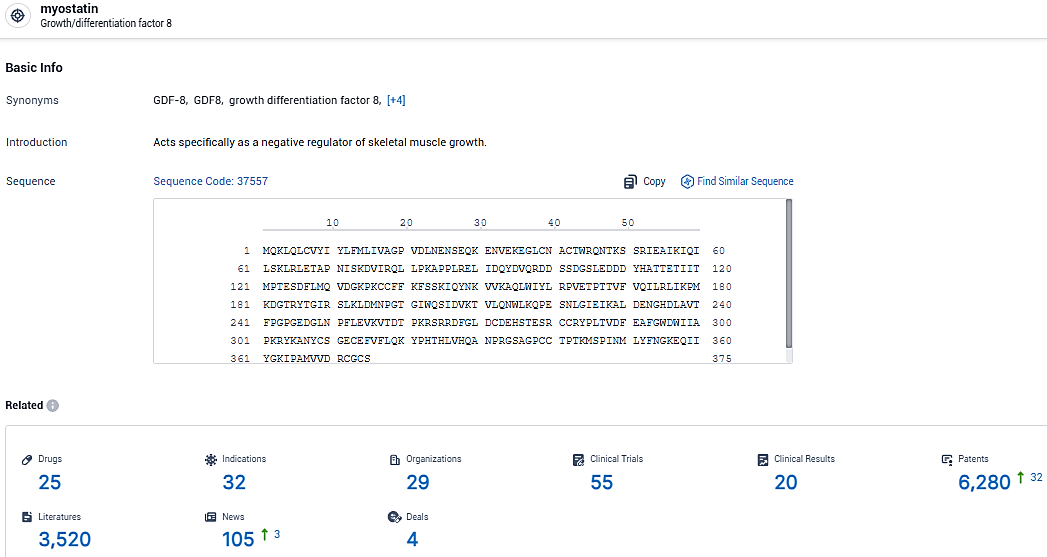
According to the data provided by the Synapse Database, As of January 29, 2024, there are 25 investigational drugs for the myostatin target, including 32 indications, 29 R&D institutions involved, with related clinical trials reaching 55, and as many as 6280 patents.
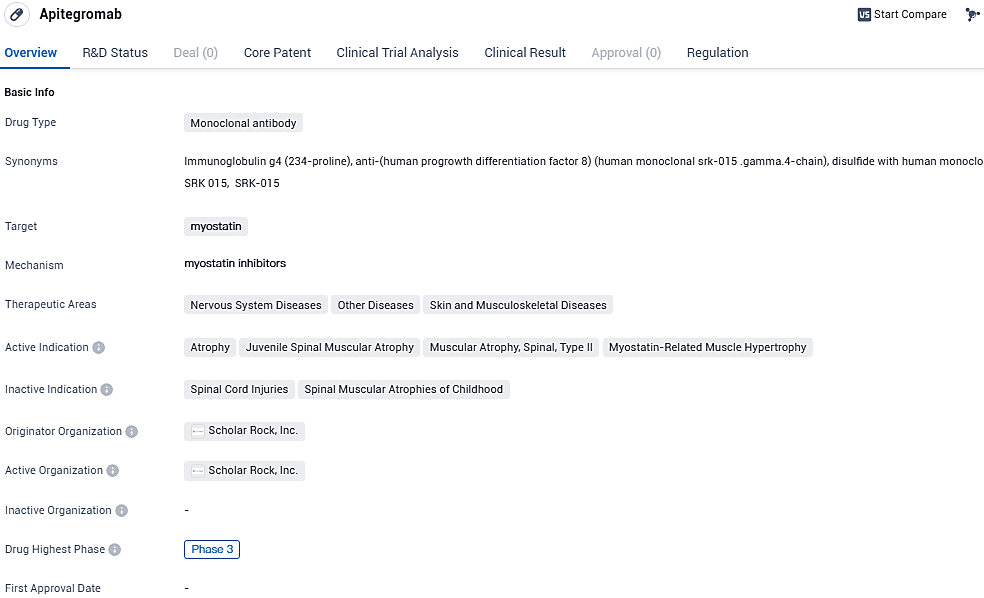
Apitegromab targets myostatin and is indicated for various conditions related to muscle atrophy and hypertrophy. Currently in Phase 3 of clinical development, the drug has received regulatory designations such as Rare Pediatric Disease, PRIME, Fast Track, and Orphan Drug. These designations highlight the drug's potential to address unmet medical needs and expedite its development and review process.