Unlock the Power of Synapse: A Guide to Searching Cyclobenzaprine
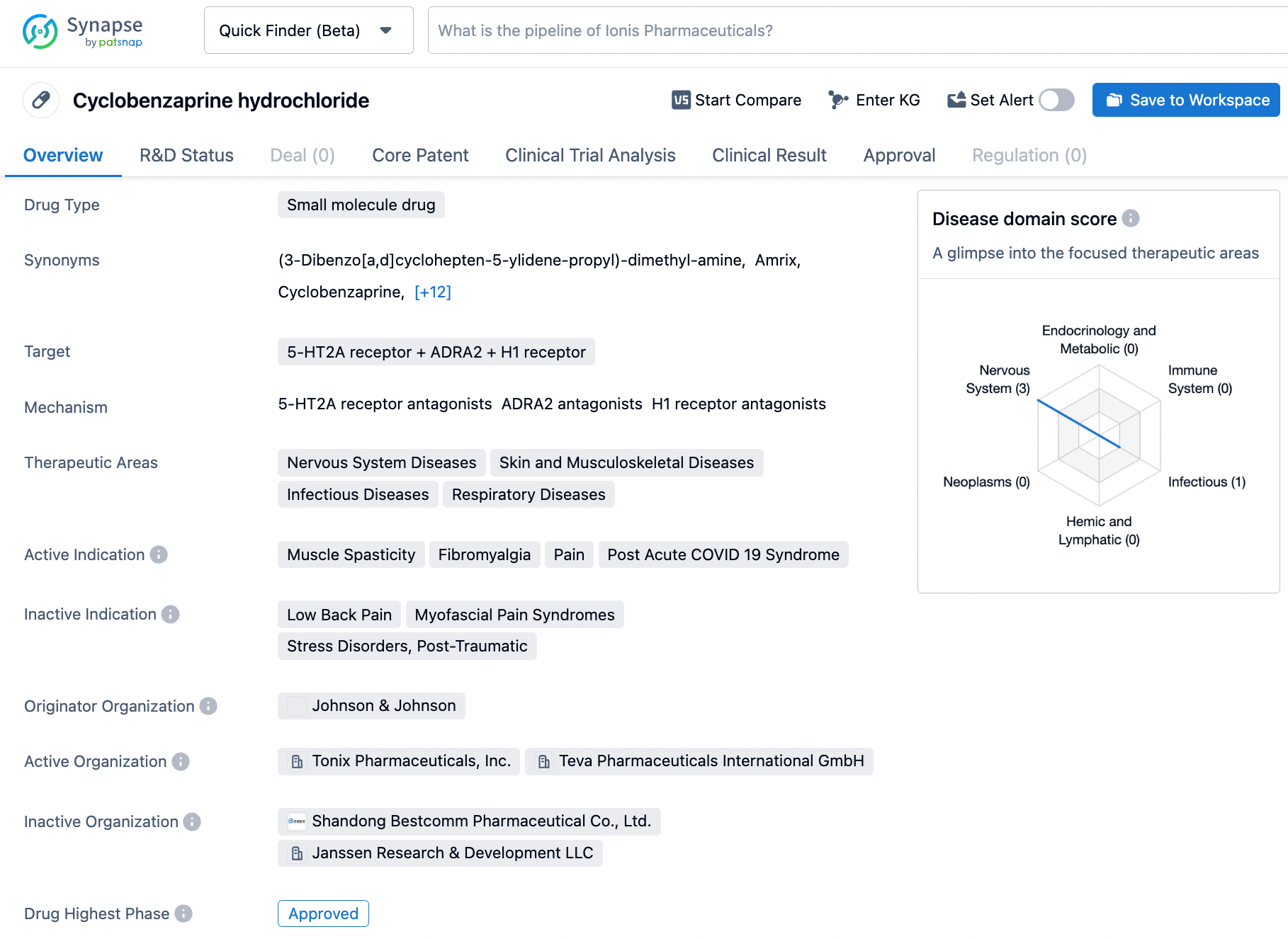
Cyclobenzaprine is a small molecule drug that antagonizes ADRA2, 5-HT2A receptor, and H1 receptor, and has been approved for the treatment of muscle spasticity, fibromyalgia, stress disorders, low back pain, COVID-19, and post-COVID syndrome. Its mechanism of action reduces the excitability of motor neurons and skeletal muscles, while diminishing anxiety, pain, and inflammation. Johnson & Johnson developed the drug, first approved in 1977, but it can cause side effects like drowsiness, dry mouth, and dizziness, which requires careful monitoring by healthcare providers. Cyclobenzaprine can provide therapeutic benefits for a wide range of clinical indications, but its administration requires prudence, particularly for patients with underlying medical conditions, such as hepatic or renal impairment. Hence, healthcare providers must be well-informed regarding the patient's medical history before administering this medication. Click on the image below to begin the exploration journey of Cyclobenzaprine through the Synapse database!
You can search for the latest pharmaceutical information such as drugs, targets, patents, transactions, clinical results, etc. through the Synapse database. Come and experience it!